- Search
| Neurospine > Volume 19(2); 2022 > Article |
|
|
Abstract
Objective
To determine whether double transverse incisions could provide superior cosmetic and functional outcomes, including rates of dysphagia and dysphonia, compared with longitudinal incisions in patients undergoing anterior cervical spine surgery (ACSS) involving Ōēź 3 levels.
Methods
A total of 62 consecutive patients who underwent ACSS involving Ōēź 3 levels were included in this study. They consist of 33 with longitudinal incisions (L group) and 29 with double transverse incisions (DT group). We recorded functional outcome measures including the Bazaz score for postoperative dysphagia and the Voice Handicap Index-10 (VHI-10) for postoperative dysphonia. The Vancouver Scar Scale (VSS) and the patient and observer scar assessment scale (POSAS) were used to evaluate postoperative skin scarring.
Results
Cosmetic results, as assessed using the VSS and POSAS, were significantly better in the DT than in the L group at most follow-up time points (p < 0.01 each). Dysphagia rates were significantly lower in the DT group than in the L group during the late postoperative period from 6 months until final 2 years of follow-up (p < 0.01 each). There were no significant different results between the 2 groups in terms of dysphonia.
Although the Smith-Robinson approach using a single transverse incision is the most widely applied approach for anterior cervical spine surgery (ACSS), operations involving Ōēź 3 levels often require longitudinal incisions for better exposure [1,2]. However, longitudinal incisions with long segments of ACSS may result in higher rates of postoperative complications [3-6], including dysphagia [7] and dysphonia [8], with unsatisfied wound problems. Higher rates of complications with multilevel ACSS might be related to the type of incision. Previous studies evaluating rates of dysphagia and dysphonia after ACSS have been limited by the lack of validated, quantitative outcomes. Therefore, it is necessary to evaluate functional outcomes as a validated value. Postoperative scarring can directly affect patient satisfaction because ACSS is the surgery in spine practice for which the wound cannot be concealed by clothing or hair. Longitudinal incisions are perpendicular to the minimal skin tension line, creating more tension than transverse incisions and possibly leading to inferior cosmetic results [9]. Moreover, to our knowledge, no study has compared the cosmetic results among different incisions in multilevel ACSS involving Ōēź 3 levels.
The purpose of this study was to determine whether double transverse incisions could provide superior cosmetic and functional outcomes, including rates of dysphagia and dysphonia, when compared with longitudinal incisions in patients undergoing ACSS involving Ōēź 3 levels.
This study enrolled 62 consecutive patients who underwent ACSS involving Ōēź 3 levels between March 2013 and February 2019. The study was approved by the institutional review board of our institution. The indications of surgery were cervical spondylosis with or without disc herniation with refractory radiculopathy or progressive myelopathy. Patients were excluded if they had (1) prior anterior cervical surgery; (2) congenital CNS disease; (3) conditions other than degenerative disease, such as trauma, infection, or tumor; or (4) a single transverse incision using the Smith-Robinson approach.
All surgical procedures were performed by a single orthopedic spine surgeon, and all patients were followed up for a minimum of 24 months. Two types of surgery, anterior cervical discectomy and fusion (ACDF) and vertebral body sliding osteotomy (VBSO), were performed. VBSO is a surgical technique reported as a substitute for corpectomy [10]. In VBSO, the vertebral body is translated anteriorly to widen the spinal canal, minimizing the need for direct removal of the pathology such as ossified mass and bony spurs [11-13]. For double transverse incision, 2 transverse incisions were made parallel to LangerŌĆÖs skin line, with the bridge of the minimum 3-cm flap secured between the 2 incisions (Fig. 1). To secure all cervical levels from C2 to T1, the upper incision was made at the C3ŌĆō4 level and the lower incision at the C6ŌĆō7 level. Platysma cutting is made parallel to the incision line in the L group, and slightly transverse in the DT group. An anterior cervical plate could be inserted into one incision, and screws could be inserted through both the upper and lower incisions (Fig. 2).
PatientsŌĆÖ demographic characteristics, including gender, age, and comorbidities, such as diabetes and hypertension, as well as surgical characteristics, including type of incision, type of surgery, level of surgery, lower level of surgery (C6, C7, T1), upper level of surgery (C3, C4), duration of surgery, and estimated blood loss, were obtained from electronic medical records. Recorded postoperative adverse events included dural tear, infection, pseudarthrosis, and skin problems.
Swallowing difficulty was assessed by contrast esophagography on postoperative day 3. Abnormal findings, including aspiration, stricture, achalasia, and spasm, were recorded. If necessary, rehabilitation and Otorhinolaryngology doctors were consulted to evaluate and manage dysphagia and dysphonia. A video fluoroscopic swallowing study (VFSS) was performed if esophagography showed abnormal results or if dysphagia persisted, with abnormal VFSS results recorded using the penetration aspiration scale (PAS).
Dysphagia and dysphonia were assessed using patient-reported outcome measures at 1, 3, 6, 12, and 24 months postoperatively. Postoperative dysphagia was determined according to the Bazaz classification, and postoperative dysphonia according to the Voice Handicap Index-10 (VHI-10). The Bazaz score graded dysphagia as none, mild, moderate, and severe [3,14]. None indicated that the patient experienced no episodes of swallowing difficulty with either liquids or solids. Mild indicated no difficulty in swallowing liquids and only some difficulty with solids. Moderate indicated no (or rare) difficulty in swallowing liquids and occasional difficulty with specific solids. Severe indicated no (or rare) difficulty in swallowing liquids and frequent difficulty with most solids. The VHI-10 is a shortened, 10-item version of the VHI, with the 2 showing a high degree of correlation (r > 0.90, p = 0.01) [15,16]. The VHI-10 contains 10 questions that subjectively assess dysphonia (Table 1). Scores on the VHI-10 ranged from 0 to 40 [15-17].
Skin scarring was evaluated at 3, 6, 12, and 24 months postoperatively. Medical photographs were taken of each patientŌĆÖs skin scar at each postoperative visit. Cosmetic results were evaluated using the Vancouver Scar Scale (VSS) and the patient and observer scar assessment scale (POSAS) [18,19]. The VSS rated scars according to 4 parameters: vascularity, pigmentation, pliability, and height. Each parameter contained ranked subscales, with total scores ranging from 0 to 13. Patients and observers rated scars on the POSAS blindly on the same day. The observer component of the POSAS consisted of 6 parameters: vascularity, pigmentation, thickness, relief, pliability, and surface area, with each parameter consisting of several categories. The patient component of the POSAS also consisted of 6 parameters: pain, itchiness, color, stiffness, thickness, and irregularity. Each parameter was rated on a 10-point scale, with 1 representing near-normal skin and 10 representing the worst scar imaginable. Patients with unsatisfactory cosmetic results after ACSS were referred to a plastic surgeon for scar revision.
Statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Time-dependent data were analyzed by repeated-measures analysis of variance, followed by post hoc comparisons of patients with longitudinal (L group) and double transverse (DT group) incisions. Bonferroni adjustments, including all pairwise comparisons within a specific model, were applied to p-values to account for multiple testing. Post hoc comparisons were performed between the main effects of all pairs of time points. The multiple imputations method with regression model was used for missing data. Statistical significance was set at p < 0.05.
Sixty-two patients were evaluated in the present study, including 33 with longitudinal incisions (L group) and 29 with double transverse incisions (DT group). All patients were followed for Ōēź 24 months, with mean follow-up of patients in the L and DT groups being 38.3 ┬▒ 16.40 and 37.7 ┬▒ 13.79 months, respectively, during which their functional and cosmetic outcomes were evaluated. The demographic characteristics, medical comorbidities, and surgical variables of the 2 groups are presented in Table 2. These 2 groups differed significantly in upper cervical level (p = 0.01). Of these 62 patients, 30 underwent ACDF, 31 underwent VBSO, and 1 underwent VBSO with ACDF. Barium esophagography on postoperative day 3 showed aspiration in 5 of 33 patients in the L group, with VFSS in 3 of these 5 patients showing PAS scores of 6, and only 1 confirmed as having vocal cord palsy. Barium esophagography showed aspiration in 2 of the 29 patients in the DT group, with 1 patient having a PAS score of 6 and none being diagnosed with vocal cord palsy. Rates of complications, including dural tears, infections, and pseudarthrosis, did not differ between the 2 groups (Table 3).
Dysphagia was assessed using the Bazaz score, and significantly improved over time in both the DT and L groups (p < 0.01 each). The Bazaz scores were significantly lower in the DT than in the L group at 6, 12, and 24 months postoperatively (p < 0.01 each). However, the differences were insignificant at 1 and 3 months (Fig. 3A, Table 4). Dysphonia assessed using the VHI10 also improved significantly over time in the DT (p < 0.01) and L (p < 0.01) groups, but the difference between the groups was not statistically significant (Fig. 3B, Table 4). Cosmetic results, as assessed using the VSS, showed that cosmesis was significantly better in the DT than in the L group at all follow-up time points (p < 0.01 each). Similarly, cosmetic results assessed using the POSAS showed significantly superior results in the DT compared with the L group at all time points except at 24 months (p < 0.01 for 3, 6, and 12 months) (Fig. 3C, D; Table 4). In addition, 7 patients in the L group (21.2%) showed severe longitudinal skin tenting along the scar, whereas none of the patients in the DT group showed a tenting sign along the scar (Table 3, Fig. 4). Moreover, patients with tenting scars complained of restricted neck extension motion. Three patients in the L group (9.1%) needed scar revision due to unsatisfactory cosmetic results after ACSS. There was no skin necrosis in DT group. Although we did not separately investigate the sensory change of the flap area, no patient complained of numbness when the medical chart was reviewed.
This study demonstrated that DT incision was better than longitudinal incision for ACSS of more than 3 levels in terms of dysphagia and cosmetic results. Proper incisions for multilevel ACSS are crucial for appropriate exposure and better functional and cosmetic outcomes. For long segments ACSS, the surgeon might choose between longitudinal and double transverse incisions.
As more patients return to the workforce and social activities after ACSS, cosmetic results may affect their quality of life [20,21]. Inferior skin scarring at the anterior neck negatively affects self-esteem and can lead to anxiety and depression [22]. However, few studies to date have assessed cosmetic outcomes after ACSS. To our knowledge, this is the first study to compare cosmetic results of double transverse incisions with longitudinal incisions for multilevel ACSS (Ōēź 3 levels). The VSS was the first validated and remained one of the most widely used scar scales [18,19]. The POSAS is a reliable and validated scar assessment scale and includes subjective evaluations by patients [18,23]. The VSS may be susceptible to observer bias, underestimating skin scarring after surgery. In addition to surgeonŌĆÖs bias, patients may be subject to a social desirability bias, as they may not want to disappoint the surgeon [20]. To control for these biases, skin scarring after multilevel ACSS was evaluated by the VSS and POSAS in this study. On both scales, the DT group showed better results than the L group. The lack of significant difference between the 2 groups on the POSAS only at 24 months may be due to improvements in patient opinions of their scars [24]. These cosmetic results agree with previous studies of postoperative linear scars. In a double transverse incision, the skin is incised parallel to the tension line, minimizing wound contraction and providing better cosmetic outcomes than longitudinal incisions [9]. The skin is maximally extensible perpendicular to the tension line, minimizing tension when incisions are made along the tension line [25]. Parallel cutting to the platysma muscle fiber has superior cosmetic results with lower rates of puckering [26]. Beyond scoring results, most patients in the DT group were satisfied with their cosmetic results. On the other hand, complications including wound revision surgery and restricted motion of neck extension in L group decreased patient quality of life after ACSS.
Higher rates of dysphagia and dysphonia have been reported in patients undergoing Ōēź 3-level ACSS than single or 2-level ACSS [3,4,27,28]. This study hypothesized that the DT group would show better outcomes than the L group in dysphagia and dysphonia after multilevel ACSS. Dysphagia rates were significantly lower in the DT than in the L group from 6 months postoperatively to 24 months of final follow-up. These findings indicated that DT group showed a better outcome in chronic postoperative dysphagia than L group. We examined the possible reasons for the low rates of dysphagia after long segments of ACSS using a double transverse incision as follows. Dysphagia can be classified according to time and by various causes, and it can also be caused by extrinsic compression in the chronic stage [3,14,29]. In the DT group, skin tenting with fibrosis and platysma puckering [26] occur less than in the L group because the direction of the incision is parallel to the skin crease and the muscle fiber in the platysma [9,21,30]. The effect of extrinsic compression due to this fibrotic tissue formation may affect the difference in the incidence of dysphagia.
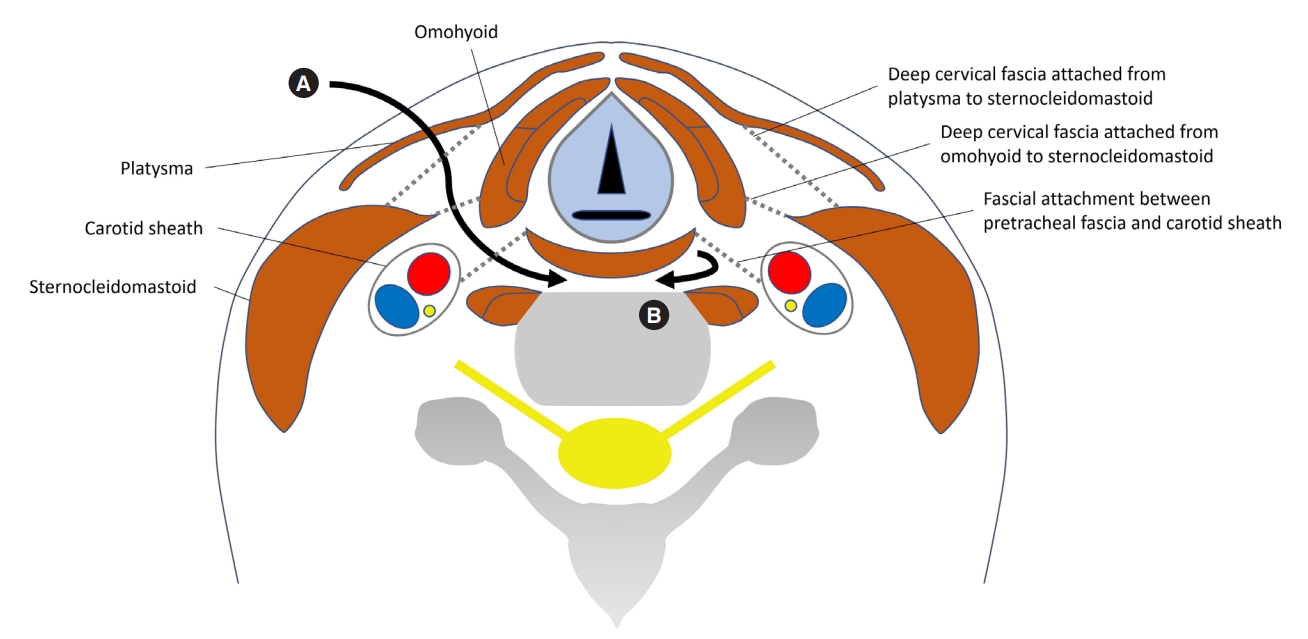
DT incision may also be advantageous over L incision for deeper level dissection. For deep cervical fascia dissection, blunt dissection is commonly used to prevent unwanted nerve injury due to sharp dissection [1]. However, in surgical approach exposure process for long segments more than 3 levels, it would be difficult to complete the exposure simply by blunt dissection; under this situation, sharp separation is inevitable [31]. Therefore, excessive blunt dissection cannot be performed in the upper and lower ends, and sharp dissection is necessary. Because the recurrent laryngeal nerve, internal branch of superior laryngeal nerve (SLN), superior thyroid artery, and superior laryngeal artery run between the middle layer of deep cervical fascia (strap muscle fascia) and the carotid sheath, the probability of an injury causing permanent dysphagia is increased during sharp dissection of the deep cervical fascia [31-34]. On the other hand, with DT incision, less than 2 levels at upper and lower incisions are to be exposed, so sharp dissection is rarely required and most cases are resolved with blunt dissection. These differences seem to be related to differences in the incidence of permanent dysphagia. Parallel incision with nerve pathway may also explain the advantages of double transverse incisions over longitudinal incisions in reducing nerve injuries that result in dysphagia. In the upper cervical level, hypoglossal nerve travels transversally at the level of C2ŌĆō3, and the SLN travels transversally and the internal branch of the SLN travels similarly from the investing fascia surrounding the carotid sheath to the thyrohyoid membrane at the C3ŌĆō4 level [32,35-37]. Compared to the longitudinal incision, a double transverse incision can provide transverse visibility and field space of the upper cervical level. Previous studies have reported that thorough dissection parallel to the nerve pathway could lower the possibility of nerve injuries in excessive retraction and unintentional ligation [35,36].
In addition, using DT incision has the advantage that it is not necessary to dissect all levels of deep cervical fascia to reach the prevertebral level. There is a surgical method for fracture called minimally invasive plate osteosynthesis (MIPO) [38]. MIPO is an advantageous method making an incision only in the upper and lower parts without opening all parts during the plating process to increase vascularity of the fracture site, thus improving functional outcome and cosmesis. Using DT incision, the middle part can be spared since it is possible to reach the prevertebral level using the space of the upper and lower incision levels (Fig. 5). At the level between C3ŌĆō4 and C6ŌĆō7, the path of the external SLN is variable, suggesting that a direct longitudinal dissection may increase the risk of unintentional damage to its branches because perpendicular direction of incision to nerve pathway. Without bridge flap invasion, double transverse incision could avoid incidental injury to superficial branches of SLN. Considering that most nerve injuries that cause dysphagia occur during the dissection of the deep cervical fascia [1,31,33,34], sparing the middle part of cervical level can be advantageous by using DT incision. Whereas, rates of dysphonia, as measured by VHI-10 scores, did not differ significantly between the 2 groups, perhaps due to the relatively low rates of dysphonia after ACSS. This result suggested that the incidence of dysphonia after multilevel ACSS might be more affected by number of levels or duration of surgery than type of incision.
This study had several limitations. First, it was a non-randomized and retrospective analysis involving relatively few patients, making it underpowered. Second, dysphagia and dysphonia were not measured preoperatively, preventing determination of improvements over baseline. Third, the surgeons determined the incision, which may have caused a selection bias. Moreover, the VBSO is known to have a lower complication rate than corpectomy, but it is not yet familiar to all surgeons. Therefore, it may be helpful to consider a double transverse incision while preparing a long-level ACDF, but for VBSO, we recommend to try a double transverse incision after gaining sufficient surgical experience with its technique. Finally, the different surgical types (VBSO versus ACDF) may have affected the results of postoperative dysphagia more significantly than the incision type. Comparing 2 incisions within both types of surgery can be too heterogenous. However, surgery types between 2 groups did not differ significantly. Therefore, we concluded that the incision type may have a considerable effect on the results of postoperative dysphagia after long level ACSS.
In conclusion, present study focused on ACSS involving Ōēź 3 levels, and analyzed the association of incision type with dysphagia, dysphonia, and skin cosmesis. A double transverse incision can be used when performing ACSS involving Ōēź 3 levels, possibly providing better cosmesis and lower rates of persistent dysphagia than a longitudinal incision.
NOTES
ACKNOWLEDGEMENTS
This study was presented in the 48th Annual Meeting of the Cervical Spine Research Society (Las Vegas, Dec, 2020).
Fig.┬Ā1.
Incision lines for anterior cervical spine surgery. (A) Longitudinal incision. (B) Double transverse incision.
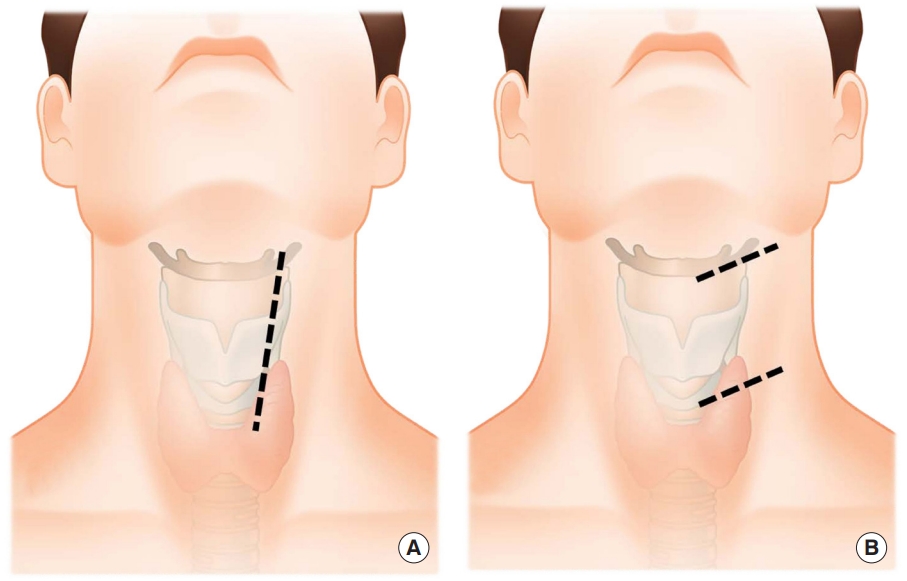
Fig.┬Ā2.
Intraoperative (A) and immediate postoperative (B) medical photos of a double transverse incision, showing a 3-cm bridge flap between the 2 incisions.
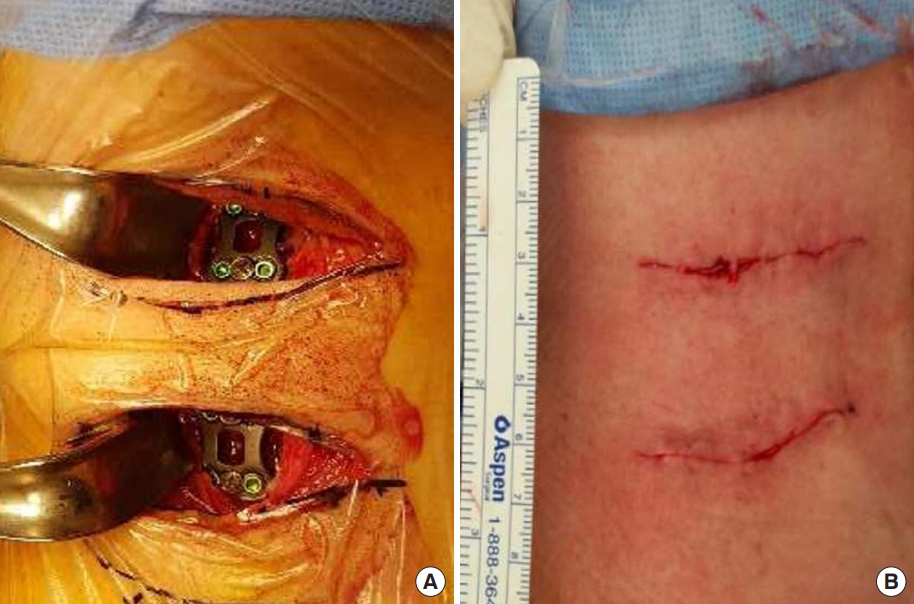
Fig.┬Ā3.
Functional results of dysphagia and dysphonia and cosmetic outcomes after anterior cervical spine surgery using patient-reported outcome. Bazaz scores (A), VHI-10 scores (B), Vancouver Scar Scale (VSS) (C), and patient and observer scar assessment scale (POSAS) (D). ACSS, anterior cervical spine surgery; VHI-10, Voice Handicap Index-10. L, longitudinal; DT, double transverse; POD, postoperative day. *Statistically significant difference at each time point (p < 0.01).
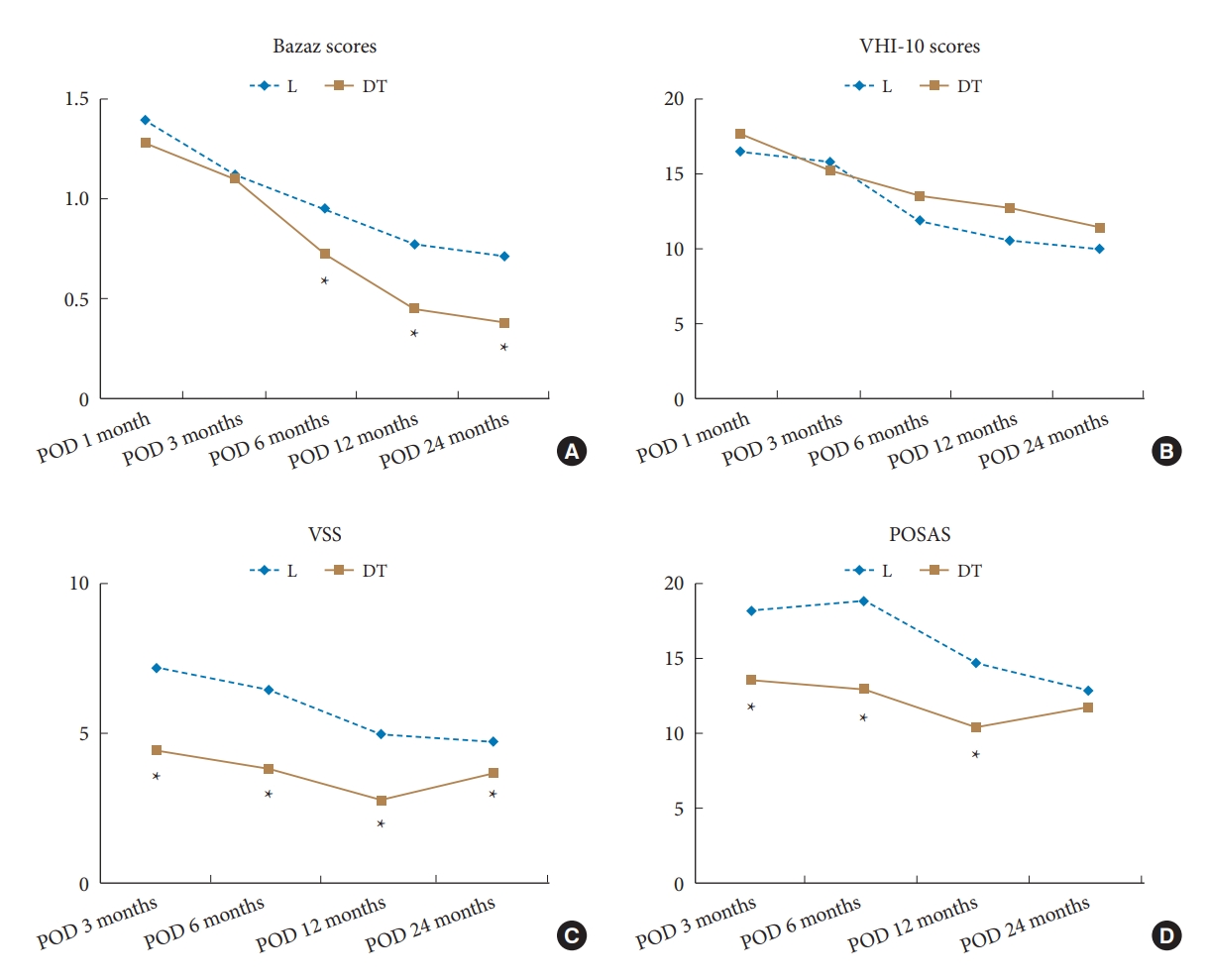
Fig.┬Ā4.
Skin scarring 24 months after anterior cervical spine surgery using a longitudinal incision (A) and a double transverse incision (B).

Fig.┬Ā5.
Illustration of axial cut in midsection spared in double transverse incision. Surgical routes are expressed with longitudinal incision (A) and double transverse incision (B). Skin incision, platysma cutting, and dissection of deep cervical fascial layers are performed in midsection using longitudinal incision. In double transverse incision, the dissection process described above can be omitted to reach the prevertebral fascia using the space at the upper and lower cervical levels.
Table┬Ā1.
VHI-10 questionnaire
Table┬Ā2.
Demographic and clinical characteristics of study patients
| Characteristic |
Incision type |
p-value | |
|---|---|---|---|
| L (n=33) | DT (n=29) | ||
| Sex, male:female | 21:12 | 16:13 | 0.49 |
| Age (yr) | 59.1 ┬▒ 13.3 | 62.7 ┬▒ 11.6 | 0.41 |
| DM | 9 (27.2) | 6 (20.7) | 0.54 |
| HTN | 10 (30.3) | 6 (20.7) | 0.38 |
| No. of involved levels | 0.07 | ||
| ŌĆā3 | 21 | 12 | |
| ŌĆā4 | 12 | 16 | |
| ŌĆā5 | - | 1 | |
| Upper cervical level | 0.01* | ||
| ŌĆāC3 | 18 | 24 | |
| ŌĆāC4 | 15 | 5 | |
| Lower cervical level | 0.78 | ||
| ŌĆāC6 | 7 | 7 | |
| ŌĆāC7 | 25 | 21 | |
| ŌĆāT1 | 1 | 1 | |
| Operation | 0.07 | ||
| ŌĆāACDF | 12 | 18 | |
| ŌĆāVBSO | 20 | 11 | |
| ŌĆāACDF+VBSO | 1 | - | |
| Duration of surgery (min) | 209.5 ┬▒ 27.7 | 222.6 ┬▒ 35.4 | 0.74 |
| EBL (mL) | 142.7 ┬▒ 106.5 | 150.3 ┬▒ 114.2 | 0.91 |
Table┬Ā3.
Frequency of adverse events
| Adverse events | L (n = 33) | DT (n = 29) | p-value |
|---|---|---|---|
| Abnormal esophagogram | 5 (15.2) | 2 (6.9) | 0.351 |
| Scar revision | 3 (9.1) | 0 (0) | 0.09 |
| Skin tenting sign | 7 (21.2) | 0 (0) | < 0.01* |
| Skin necrosis | 0 (0) | 0 (0) | - |
| Infection | 0 (0) | 0 (0) | - |
| Dural tear | 1 (3) | 0 (0) | 0.34 |
| Pseudarthrosis | 6 (18.2) | 4 (13.8) | 0.577 |
Table┬Ā4.
Patient-reported outcomes
| Variable |
Incision type |
p-value | |
|---|---|---|---|
| L (n=33) | DT (n=29) | ||
| Bazaz scores | |||
| ŌĆāPOD 1 month | 1.41 ┬▒ 0.83 | 1.27 ┬▒ 0.87 | 0.575 |
| ŌĆāPOD 3 months | 1.13 ┬▒ 0.80 | 1.09 ┬▒ 0.84 | 0.939 |
| ŌĆāPOD 6 months | 0.96 ┬▒ 0.69 | 0.72 ┬▒ 0.87 | 0.018* |
| ŌĆāPOD 12 months | 0.75 ┬▒ 0.50 | 0.47 ┬▒ 0.60 | 0.007* |
| ŌĆāPOD 24 months | 0.69 ┬▒ 0.50 | 0.39 ┬▒ 0.63 | 0.005* |
| VHI-10 scores | |||
| ŌĆāPOD 1 month | 16.02 ┬▒ 9.32 | 17.61 ┬▒ 10.43 | 0.615 |
| ŌĆāPOD 3 months | 13.42 ┬▒ 9.00 | 17.05 ┬▒ 11.27 | 0.265 |
| ŌĆāPOD 6 months | 11.52 ┬▒ 9.05 | 13.57 ┬▒ 10.97 | 0.521 |
| ŌĆāPOD 12 months | 9.59 ┬▒ 8.57 | 12.75 ┬▒ 10.23 | 0.295 |
| ŌĆāPOD 24 months | 8.86 ┬▒ 7.50 | 11.46 ┬▒ 9.52 | 0.339 |
| VSS | |||
| ŌĆāPOD 3 months | 7.20 ┬▒ 2.64 | 4.44 ┬▒ 1.94 | < 0.0001* |
| ŌĆāPOD 6 months | 6.45 ┬▒ 2.92 | 3.82 ┬▒ 3.11 | < 0.0001* |
| ŌĆāPOD 12 months | 4.96 ┬▒ 2.71 | 2.74 ┬▒ 2.81 | < 0.0001* |
| ŌĆāPOD 24 months | 4.73 ┬▒ 2.77 | 3.67 ┬▒ 2.88 | 0.009* |
| POSAS | |||
| ŌĆāPOD 3 months | 18.17 ┬▒ 7.10 | 13.52 ┬▒ 4.34 | < 0.0001* |
| ŌĆāPOD 6 months | 18.81 ┬▒ 8.11 | 12.94 ┬▒ 5.27 | < 0.0001* |
| ŌĆāPOD 12 months | 14.74 ┬▒ 6.85 | 10.40 ┬▒ 3.92 | < 0.0001* |
| ŌĆāPOD 24 months | 12.88 ┬▒ 6.89 | 11.72 ┬▒ 7.01 | 0.247 |
REFERENCES
2. Choi SH, Kang CN. Degenerative cervical myelopathy: pathophysiology and current treatment strategies. Asian Spine J 2020;14:710-20.




3. Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976) 2002;27:2453-8.

4. Cho SK, Lu Y, Lee DH. Dysphagia following anterior cervical spinal surgery: a systematic review. Bone Joint J 2013;95-B:868-73.

5. Tervonen H, Niemel├ż M, Lauri ER, et al. Dysphonia and dysphagia after anterior cervical decompression. J Neurosurg Spine 2007;7:124-30.


6. Louie PK, Sexton AC, Bohl DD, et al. Rigid-plating and cortico-cancellous allograft are effective for 3-level anterior cervical discectomy and fusion: radiographic and clinical outcomes. Neurospine 2020;17:146-55.




7. Perez-Roman RJ, Luther EM, McCarthy D, et al. National trends and correlates of dysphagia after anterior cervical discectomy and fusion surgery. Neurospine 2021;18:147-54.




8. Mehra S, Heineman TE, Cammisa FP Jr, et al. Factors predictive of voice and swallowing outcomes after anterior approaches to the cervical spine. Otolaryngol Head Neck Surg 2014;150:259-65.



9. Wilhelmi BJ, Blackwell SJ, Phillips LG. LangerŌĆÖs lines: to use or not to use. Plast Reconstr Surg 1999;104:208-14.


10. Lee DH, Cho JH, Lee CS, et al. A novel anterior decompression technique (vertebral body sliding osteotomy) for ossification of posterior longitudinal ligament of the cervical spine. Spine J 2018;18:1099-105.


11. Lee DH, Park S, Hong CG, et al. Significance of vertebral body sliding osteotomy as a surgical strategy for the treatment of cervical ossification of the posterior longitudinal ligament. Global Spine J 2022;12:1074-83.




12. Lee DH, Park S, Lee WS, et al. Vertebral body sliding osteotomy for cervical myelopathy with rigid kyphosis. Neurospine 2020;17:640-7.




13. Lee DH, Riew KD, Choi SH, et al. Safety and efficacy of a novel anterior decompression technique for ossification of posterior longitudinal ligament of the cervical spine. J Am Acad Orthop Surg 2020;28:332-41.


14. Shriver MF, Lewis DJ, Kshettry VR, et al. Dysphagia rates after anterior cervical diskectomy and fusion: a systematic review and meta-analysis. Global Spine J 2017;7:95-103.




15. Forti S, Amico M, Zambarbieri A, et al. Validation of the Italian Voice Handicap Index-10. J Voice 2014;28:263.e17-263.e22.


16. Arffa RE, Krishna P, Gartner-Schmidt J, et al. Normative values for the Voice Handicap Index-10. J Voice 2012;26:462-5.


17. Behlau M, Madazio G, Moreti F, et al. Efficiency and cutoff values of self-assessment instruments on the impact of a voice problem. J Voice 2016;30:506.e9-506.e18.


18. Truong PT, Lee JC, Soer B, et al. Reliability and validity testing of the Patient and Observer Scar Assessment Scale in evaluating linear scars after breast cancer surgery. Plast Reconstr Surg 2007;119:487-94.


19. Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 2004;113:1960-5. discussion 1966-7.


20. Vila PM, Ramsey T, Yaeger LH, et al. Reporting of cosmesis in head and neck reconstruction: a systematic review. Otolaryngol Head Neck Surg 2019;160:573-9.



21. Cheung KM, Mak KC, Luk KD. Anterior approach to cervical spine. Spine (Phila Pa 1976) 2012;37:E297-302.


22. Bronheim H, Strain JJ, Biller HF. Psychiatric aspects of head and neck surgery. Part II: Body image and psychiatric intervention. Gen Hosp Psychiatry 1991;13:225-32.

23. Vercelli S, Ferriero G, Bravini E, et al. Cross-cultural adaptation, reproducibility and validation of the Italian version of the Patient and Observer Scar Assessment Scale (POSAS). Int Wound J 2017;14:1262-8.




24. Martin D, Umraw N, Gomez M, et al. Changes in subjective vs objective burn scar assessment over time: does the patient agree with what we think? J Burn Care Rehabil 2003;24:239-44. discussion 238.



25. Son D, Harijan A. Overview of surgical scar prevention and management. J Korean Med Sci 2014;29:751-7.




26. Agrawal A, Rao M. Transverse cervical skin incision and vertical platysma splitting approach for anterior cervical vertebral column exposure. Rom Neurosurg 2014;21:89-93.

27. Tasiou A, Giannis T, Brotis AG, et al. Anterior cervical spine surgery-associated complications in a retrospective case-control study. J Spine Surg 2017;3:444-59.



28. Teo SJ, Yeo W, Ling MZ, et al. The effect of body mass index on long-term patient-reported outcome scores after anterior cervical discectomy and fusion in an Asian population: a 2-year study. Asian Spine J 2021;15:512-22.




29. Olsson EC, Jobson M, Lim MR. Risk factors for persistent dysphagia after anterior cervical spine surgery. Orthopedics 2015;38:e319-23.


30. Chandler JR, Ponzoli VA. The use of double transverse incisions in major head and neck surgery. Ann Otol Rhinol Laryngol 1969;78:757-70.



31. Shan J, Jiang H, Ren D, et al. Anatomic relationship between right recurrent laryngeal nerve and cervical fascia and its application significance in anterior cervical spine surgical approach. Spine (Phila Pa 1976) 2017;42:E443-7.


32. Haller JM, Iwanik M, Shen FH. Clinically relevant anatomy of high anterior cervical approach. Spine (Phila Pa 1976) 2011;36:2116-21.


33. Haller JM, Iwanik M, Shen FH. Clinically relevant anatomy of recurrent laryngeal nerve. Spine (Phila Pa 1976) 2012;37:97-100.


34. Rajabian A, Walsh M, Quraishi NA. Berry's Ligament and the Inferior Thyroid Artery as reliable anatomical landmarks for the Recurrent Laryngeal Nerve (RLN): a fresh-cadaveric study of the cervical spine. The RLN relevant to spine. Spine J 2017;17:S33-9.


35. Okamoto N, Azuma S. Upper cervical anterior fusion with a particular focus on superior laryngeal nerve and hypoglossal nerve. Spine Surg Relat Res 2018;2:121-6.



36. Park SH, Sung JK, Lee SH, et al. High anterior cervical approach to the upper cervical spine. Surg Neurol 2007;68:519-24. discussion 524.



- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 7,991 View
- 290 Download
-
Journal Impact Factor 3.8
SURGERY: Q1
CLINICAL NEUROLOGY: Q1





























