3. Singh A, Tetreault L, Kalsi-Ryan S, et al. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol 2014;6:309-31.


4. Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma 2004;21:1355-70.


10. Hadley MN, Walters BC, Grabb PA, et al. Cervical spine immobilization before admission to the hospital. Neurosurgery 2002;50(3 Suppl):S7-17.


13. Snyder R, Verla T, Ropper AE. Practical application of recent advances in diagnostic, prognostic, and therapeutic modalities for spinal cord injury. World Neurosurg 2020;136:330-6.


15. Mputu Mputu P, Beauséjour M, Richard-Denis A, et al. Early predictors of neurological outcomes after traumatic spinal cord injury: a systematic review and proposal of a conceptual framework. Am J Phys Med Rehabil 2021;100:700-11.


17. Qiu Y, Chen Y, Xie Y, et al. Comparative analysis of the efficacy of early and late surgical intervention for acute spinal cord injury: a systematic review and meta-analysis based on 16 studies. Int J Surg 2021;94:106098.


21. Badhiwala JH, Wilson JR, Witiw CD, et al. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol 2021;20:117-26.


26. Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 1997;277:1597-604.


28. Matsumoto T, Tamaki T, Kawakami M, et al. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine (Phila Pa 1976) 2001;26:426-30.


36. Mojtahedzadeh M, Taghvaye-Masoumi H, Najafi A, et al. Management of hypotension and bradycardia caused by spinal cord injury. The usefulness of midodrine and methylxanthines. Iran J Pharm Res 2019;18:2131-5.


37. Khan NR, Smalley Z, Nesvick CL, et al. The use of lumbar drains in preventing spinal cord injury following thoracoabdominal aortic aneurysm repair: an updated systematic review and meta-analysis. J Neurosurg Spine 2016;25:383-93.


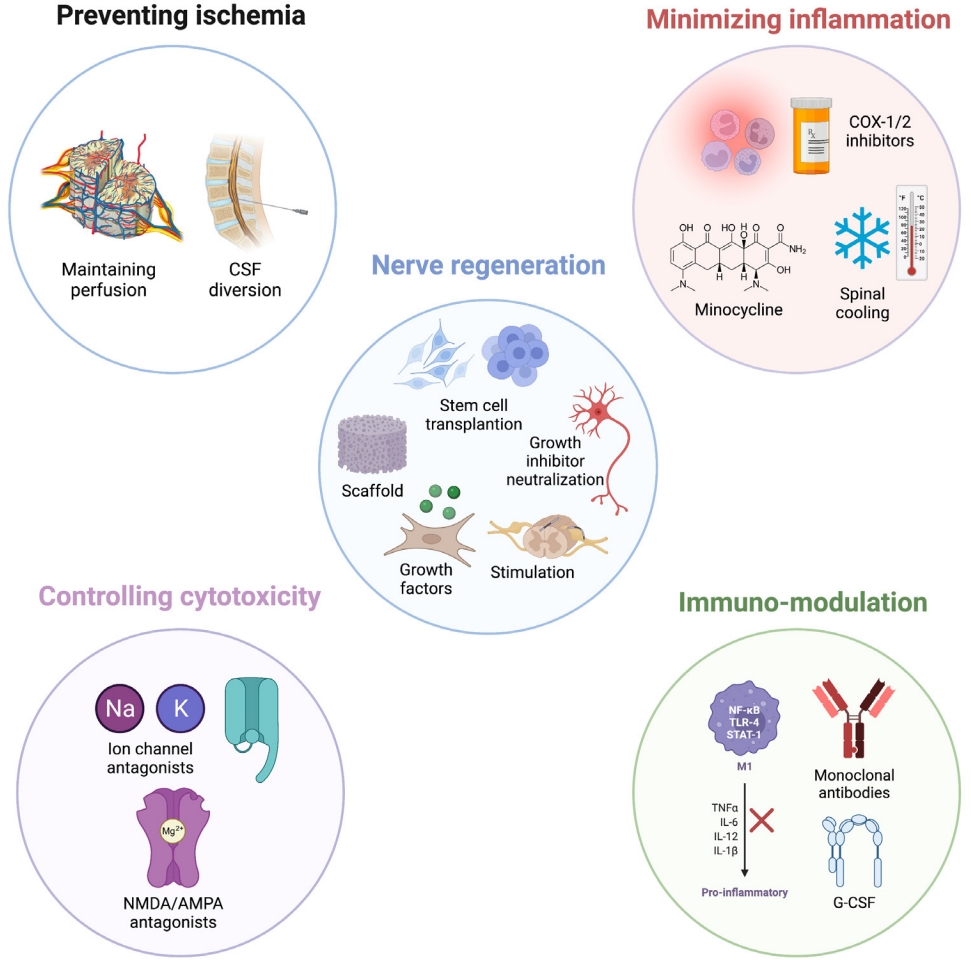
38. Martirosyan NL, Kalani MY, Bichard WD, et al. Cerebrospinal fluid drainage and induced hypertension improve spinal cord perfusion after acute spinal cord injury in pigs. Neurosurgery 2015;76:461-8. discussion 468-9.


39. Kwon BK, Curt A, Belanger LM, et al. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine 2009;10:181-93.


40. Squair JW, Bélanger LM, Tsang A, et al. Spinal cord perfusion pressure predicts neurologic recovery in acute spinal cord injury. Neurology 2017;89:1660-7.


41. Tykocki T, Poniatowski Ł, Czyż M, et al. Intraspinal pressure monitoring and extensive duroplasty in the acute phase of traumatic spinal cord injury: a systematic review. World Neurosurg 2017;105:145-52.


44. Schwartz G, Fehlings MG. Secondary injury mechanisms of spinal cord trauma: a novel therapeutic approach for the management of secondary pathophysiology with the sodium channel blocker riluzole. Prog Brain Res 2002;137:177-90.

45. Chaves RHF, Souza CC, Furlaneto IP, et al. Influence of tramadol on functional recovery of acute spinal cord injury in rats. Acta Cir Bras 2018;33:1087-94.


46. Harada N, Taoka Y, Okajima K. Role of prostacyclin in the development of compression trauma-induced spinal cord injury in rats. J Neurotrauma 2006;23:1739-49.


47. Young W, Flamm ES, Demopoulos HB, et al. Effect of naloxone on posttraumatic ischemia in experimental spinal contusion. J Neurosurg 1981;55:209-19.


48. Winkler T, Sharma HS, Stålberg E, et al. Opioid receptors influence spinal cord electrical activity and edema formation following spinal cord injury: experimental observations using naloxone in the rat. Neurosci Res 1994;21:91-101.


50. Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol 2001;166:7527-33.


51. Wells JE, Hurlbert RJ, Fehlings MG, et al. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain 2003;126(Pt 7):1628-37.


52. Squair JW, Ruiz I, Phillips AA, et al. Minocycline reduces the severity of autonomic dysreflexia after experimental spinal cord injury. J Neurotrauma 2018;35:2861-71.


53. Casha S, Zygun D, McGowan MD, et al. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012;135(Pt 4):1224-36.


54. Hansebout RR, Hansebout CR. Local cooling for traumatic spinal cord injury: outcomes in 20 patients and review of the literature. J Neurosurg Spine 2014;20:550-61.


57. Thompson CD, Zurko JC, Hanna BF, et al. The therapeutic role of interleukin-10 after spinal cord injury. J Neurotrauma 2013;30:1311-24.


59. Nishio Y, Koda M, Kamada T, et al. Granulocyte colony-stimulating factor attenuates neuronal death and promotes functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol 2007;66:724-31.


60. Derakhshanrad N, Saberi H, Yekaninejad MS, et al. Granulocyte-colony stimulating factor administration for neurological improvement in patients with postrehabilitation chronic incomplete traumatic spinal cord injuries: a double-blind randomized controlled clinical trial. J Neurosurg Spine 2018;29:97-107.


61. Koda M, Hanaoka H, Fujii Y, et al. Randomized trial of granulocyte colony-stimulating factor for spinal cord injury. Brain 2021;144:789-99.


63. Vaquero J, Zurita M, Rico MA, et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 2018;20:806-19.

64. Aghayan HR, Arjmand B, Yaghoubi M, et al. Clinical outcome of autologous mononuclear cells transplantation for spinal cord injury: a systematic review and meta-analysis. Med J Islam Repub Iran 2014;28:112.


68. Badhiwala JH, Ahuja CS, Fehlings MG. Time is spine: a review of translational advances in spinal cord injury. J Neurosurg Spine 2018;30:1-18.


75. Wu JC, Huang WC, Tsai YA, et al. Nerve repair using acidic fibroblast growth factor in human cervical spinal cord injury: a preliminary phase I clinical study. J Neurosurg Spine 2008;8:208-14.


76. Wu JC, Huang WC, Chen YC, et al. Acidic fibroblast growth factor for repair of human spinal cord injury: a clinical trial. J Neurosurg Spine 2011;15:216-27.


77. Kitamura K, Iwanami A, Nakamura M, et al. Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J Neurosci Res 2007;85:2332-42.


81. Jacobson PB, Goody R, Lawrence M, et al. Elezanumab, a human anti-RGMa monoclonal antibody, promotes neuroprotection, neuroplasticity, and neurorecovery following a thoracic hemicompression spinal cord injury in non-human primates. Neurobiol Dis 2021;155:105385.


82. Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury--a randomized, placebocontrolled trial with GM-1 ganglioside. N Engl J Med 1991;324:1829-38.


84. Angeli CA, Boakye M, Morton RA, et al. Recovery of overground walking after chronic motor complete spinal cord injury. N Engl J Med 2018;379:1244-50.


92. Wang Y, Wang J, Wang H, et al. Necrosulfonamide attenuates spinal cord injury via necroptosis inhibition. World Neurosurg 2018;114:e1186-91.