- Search
| Neurospine > Volume 19(3); 2022 > Article |
|
|
See commentary "Multimodal Repair of Spinal Cord Injury With Mesenchymal Stem Cells: An Editorial Perspective" in Volume 19 on page 630.
Abstract
Spinal cord injury (SCI) is a result of a devastating injury to the central nervous system. Currently, there is no effective treatment available for these patients. The possible use of mesenchymal stem cell (MSC)-based treatment for SCI has been the focus of extensive investigations and is increasingly moving from the bench to bedside. Both experimental observations and clinical studies have shown the safety and efficacy of MSCs in managing SCI. However, the exact mechanism by which MSCs contribute to the repair of the injured spinal cord remains to be elucidated. In this review, we aim to summarize current research findings about the role of MSCs in improving complex pathology after SCI. MSCs exert a multimodal repair mechanism targeting multiple events in the secondary injury cascade. Our recent results showing the perineurium-like differentiation of surviving MSCs in the injured spinal cord may further the understanding of the fate of transplanted MSCs. These findings provide fundamental support for the clinical use of MSCs in SCI patients. Under experimental conditions, combining novel physical, chemical, and biological approaches led to significant improvements in the therapeutic efficacy of MSCs. These findings hold promise for the future of cell-based clinical treatment of SCI.
Spinal cord injury (SCI) is a devastating central nervous system condition, caused by direct physical impact. The lack of effective treatment causes significant public concerns. Following SCI, patients live with some degree of permanent disability and several complications can arise. According to the World Health Organization, the global estimated prevalence of SCI is 40 to 80 cases per million population. For example, in the United States, there were 17,810 new cases reported in 2020, with a total of 294,000 Americans living with SCI [1]. The provision of effective treatment for SCI remains an unmet medical need [2]. Alleviating the suffering of these patients and reducing the need for their extensive care is a challenge to society.
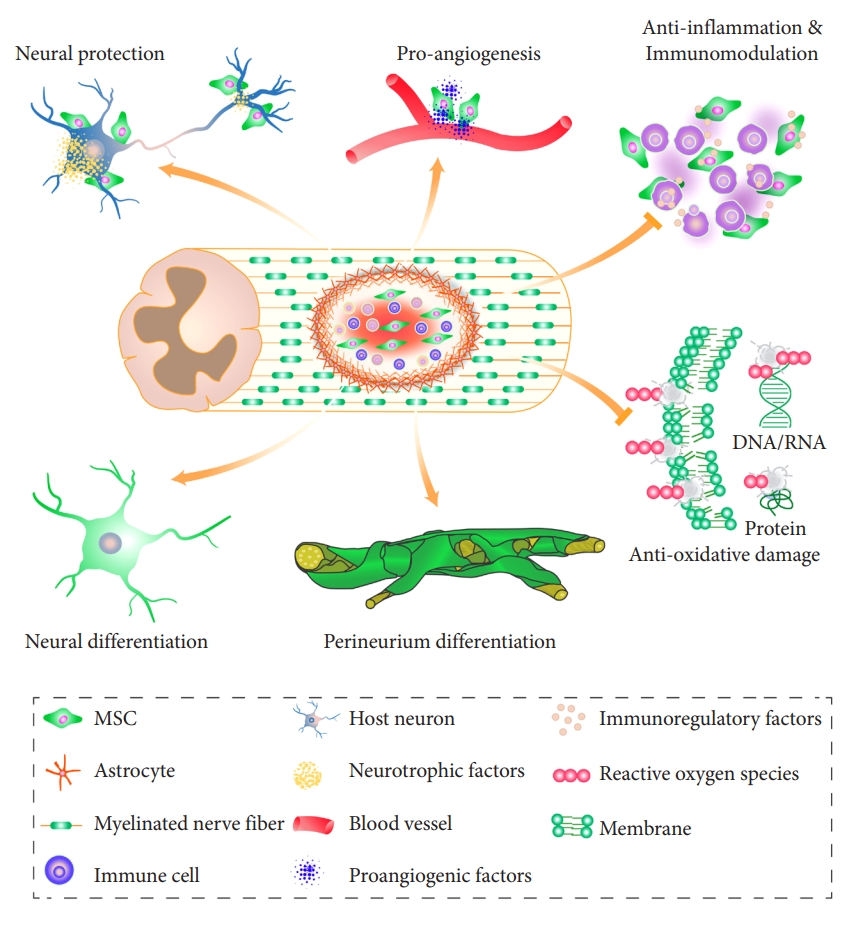
The recent emergence of stem cell-based tissue engineering is a promising strategy for SCI repair. Amongst the possible candidate seed cells, mesenchymal stem cells (MSCs) are the most extensively studied both in basic science experiments and in translational research. According to the Web of Science database, over 2,200 publications appeared on this topic and the National Library of Medicine database contains 40 registered clinical studies in various countries, including China (10), the United States (7), and the Republic of Korea (3). Due to their immunomodulatory, anti-inflammatory, and pro-angiogenic properties, MSCs show a strong potential to promote tissue repair [3-5], with ongoing registered clinical trials [6,7]. Despite this extensive work, the mechanism that allows MSCs to repair SCI remains to be elucidated. Recently, we described a novel mechanism, where MSCs differentiate into perineurium-like sheaths to protect neural tissue damage [8]. It seems that MSCs provide a multimodal repair, addressing several events in the multidimensional pathophysiology seen after SCI. In this review, we summarize the possible roles of MSCs in the treatment of SCI, highlighting multiple mechanisms that underlie their therapeutic benefits.
A full understanding of the pathophysiological events that follow SCI is necessary to identify effective treatments. The primary injury is the physical impact affecting the spinal cord directly as a result of a traffic accident, fall, sports accident, or other violent events. This initial impact can displace bone fragments and components of the intervertebral discs. Alternatively, ligaments bruise or tear into the spinal canal. These mechanisms lead to the penetration, contusion, or compression of the cord tissue [9]. The primary injury activates a rapid cascade of secondary pathophysiological changes that are collectively referred to as secondary injury. There are three early phases of secondary injury: the acute phase (within 48 hours), the subacute phase (2–14 days), and the intermediate phase (14 days–6 months). These are followed by a chronic phase (beyond 6 months) [10]. While characteristic pathophysiological changes occur during each of these phases, these are not entirely independent, but represent a step in a complex series of interlinked events. The main pathophysiological changes after SCI include hemorrhage, ischemia-reperfusion injury, alterations in ion distribution, excitotoxicity, oxidative damage, and axonal degeneration [11]. The homeostasis of the spinal cord internal microenvironment is completely disrupted after injury, leading to a cascade of pathophysiological changes. Despite our current insight into the biochemical reactions and pathways involved in the progression of SCI, much remains to be explored. A fuller understanding of the pathophysiology after injury will help identify potential avenues to improve current therapies, and potentially create new approaches that may promote spinal cord tissue regeneration.
In the 1970s a new type of adherent stromal cell was isolated and cultured from the bone marrow [12]. These cells exhibited fibroblast-like morphology and were multipotent cells that had the capacity to self-renew. Importantly, these cells could differentiate into a variety of cell types, including osteogenic, adipogenic, chondrogenic, and myogenic mesenchymal lineages in vitro [13-15]. Based on these characteristics, they were referred to as “mesenchymal stem cells” by Caplan in 1991 [16]. MSCs adhere to plastic under standard culture conditions and express CD90, CD105, and CD73. At the same time, they are CD45, CD34, CD14, CD11b, CD79α, or CD19 negative [17]. To date, no single marker has been identified that could distinguish MSCs from other cell types. Subsequent studies have shown that MSCs can be isolated from a variety of tissues, including bone marrow, umbilical cord, dental pulp, and adipose tissue [18]. The ability to establish MSCs from such a wide range of tissues boosted their potential for clinical applications, as these cells could be obtained without serious ethical concerns. Furthermore, MSCs are considered to be immune-privileged, because of their low immunogenicity. They express very low levels of MHC class I molecules and are completely devoid of MHC class II. There is growing evidence suggesting that MSCs can suppress immune responses [19]. Moreover, clinical studies showed the safety of MSC transplantation, excluding the risks of tumor formation [20]. Apart from proving their safety, studies have also demonstrated that in SCI MSCs do not act via a single mechanism but promote a multimodal repair. This multitude of repair mechanism can be regarded as the functional multipotency of MSCs [21-23], suggesting that they have the capacity to simultaneously treat multiple aspects of the complex pathology that characterizes SCI.
MSCs are potent inducers of angiogenesis and vasculogenesis when transplanted into tissues. This property has been observed under several conditions, including cardiovascular disease [24], wound healing [25], and SCI [26]. Reestablishing the blood supply is a critical requirement for successful tissue regeneration. The molecular mechanisms promoting angiogenesis by MSCs involve the secretion of the tissue inhibitor of metalloproteinase-1, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), platelet-derived growth factor, interleukin (IL)-6, and IL-8 [27,28]. Apart from the secretion of these cytokines, MSCs also contribute to blood vessel formation via additional mechanisms. These include providing physical contacts supporting growth and the production of components of the extracellular matrix (ECM). In a previous study, we found several transplanted MSCs surrounding newly formed blood vessels while others encircled tightly the epithelial layer, forming pericytes in the injured spinal cord. MSCs surrounding these structures expressed hypoxia-inducible factor 1-alpha and VEGF, and deposited fibronectin (FN) around the blood vessel inside [5]. This topology may contribute to synergistic effects, promoting the formation and maturation of blood vessels after SCI. This angiogenic nature of MSCs may counteract the effects of tissue ischemia developing after injury. By improving blood supply, MSC transplants promote the delivery of nutrients and oxygen necessary for tissue regeneration.
MSCs can suppress the activity of a variety of immune cells, including T and B lymphocytes, neutrophils, monocytes, and macrophages [29]. MSCs can strongly inhibit the proliferation of mitogen or alloantigen-activated T lymphocytes [30,31], irrespective of whether the T cells were autologous or allogeneic [32,33]. MSCs were also able to modulate immune responses by interacting with regulatory T cells (Treg), involved in the maintenance of immune homeostasis and self-tolerance [34]. Evidence suggests that MSCs can increase the number of Treg cells while simultaneously improving their immune suppressive action [35,36]. Key molecules involved in this interaction include MSC-derived soluble factors, such as prostaglandin E2 (PGE-2), transforming growth factor (TGF)-β1, indoleamine 2,3-dioxygenase (IDO), and HGF [37-40]. Recent studies have also shown that MSCs can inhibit B cells by arresting their proliferation at the G0/G1 phase of the cell cycle [41]. Furthermore, MSCs alter B-cell chemotaxis by significantly down-regulating the expression of CXCR4, CXCR5, CCR7, and their ligands, CXCL12, and CXCL13 [42]. It was also demonstrated that MSCs can directly inhibit the transformation of B cells into plasma cells [43]. The key molecules involved in this inhibition include interferon (IFN)-γ and B-cell activating factor [44]. MSCs can rescue resting and IL-8-activated neutrophils from apoptosis by constitutively releasing IL-6 in vitro [45]. They can also sustain and amplify the function of neutrophils via endogenously produced IL-6, IFN-β, and granulocytemacrophage colony-stimulating factor [46]. MSCs seem to drive macrophage polarization towards the anti-inflammatory M2 phenotype through the suppression of nuclear factor-kappa B p65 and the activation of STAT3 pathways [47]. This extensive immunosuppressive and regulatory action of MSCs has attracted considerable interest for potential clinical use. The infiltration of inflammatory cells into the injured SCI generates a microenvironment that is detrimental to recovery. The anti-inflammatory and immunoregulatory effects of MSCs may provide a promising approach to alleviate this neuroinflammation.
There is a view that the therapeutic effect of MSCs transplantation does not occur via direct cell replacement, but through the modulation of the host microenvironment [48]. Indeed, the nutritional activity of MSC has been widely confirmed and has become the focus of research in the treatment of several diseases [49]. MSCs secrete a variety of growth factors, neuroprotective cytokines, and chemokines in an autocrine or paracrine manner. These include HGF, VEGF, fibroblast growth factor, brainderived neurotrophic factor (BDNF), and nerve growth factor (NGF). All of these factors have been implicated in supporting the regeneration of damaged spinal cord tissues [50,51]. In addition, our team found that transplanted MSCs can also deposit FN, a well-known component of the ECM necessary for axonal growth. MSCs can also secrete Laminin and TGF-β in the injured spinal cord to promote the recovery [52]. Altogether, these changes result in a microenvironment that is conducive to regeneration [53].
Recently, an increasing number of studies confirmed that MSCs exert part of their therapeutic efficacy by secreting exosomes. These specialized membrane-coated nano-sized vesicles are secreted in large quantities by MSCs [54]. Exosomes contain biologically active molecules, various proteins, mRNA, transfer RNA, long noncoding RNAs, microRNAs, and even mitochondrial DNA. They affect cellular function via different routes [55]. Apart from MSCs a variety of cell types, including immune cells, can produce exosomes [56,57]. However, MSC-derived exosomes have unique characteristics; they exhibit immunomodulatory properties [58], elicit anti-inflammatory responses, and promote angiogenesis [59]. These actions of MSCs-derived exosomes replicate functions that the MSCs themselves provide [60]. Their natural cell membrane packaging protects the content of the exosomes against systemic degradation, making them suitable for systemic administration in living organisms [61,62]. Utilizing this feature attempts have been made to use MSC-derived exosomes for the treatment of SCI. Liu et al. [63] found that exosomes from bone-derived MSCs can repair traumatic SCI by suppressing the activation of A1 neurotoxic reactive astrocytes. Intravenously delivered MSC-derived exosomes can repair SCI by targeting M2-type macrophages at the site of injury [64]. The systematic administration of exosomes from MSCs can reduce apoptosis and inflammatory response, promote angiogenesis and functional recovery after SCI [65].
Like other stem cells, MSCs have the potential to differentiate into a range of tissues. As progenitors of a mesenchymal lineage, they intrinsically differentiate into mesoderm-derived tissues such as chondrocytes, osteocytes, and adipocytes [66]. Whether MSCs can be reprogrammed to transdifferentiate into neurons remains controversial [67]. Decades ago, a number of studies suggested that such transdifferentiation was possible under specific conditions. In early studies of human MSCs, a chemical induction formula, containing a combination of dimethylsulfoxide (DMSO), butylated hydroxyanisole (BHA) and β-mercaptoethanol (BME) yielded cells that phenotypically appeared neuron-like [68]. However, this ‘neuronal differentiation’ of MSCs after chemical induction was an artifact due to cytotoxic cell changes. ‘Chemical induction,’ by BME, BHA, and DMSO, detergents, high sodium concentration, and extreme pH values, can shrink the cell bodies of MSCs within a few hours, leaving them with a neuronlike morphology. This change may even be accompanied by an increase in the level of neuronal markers, such as neuron-specific enolase and neuronal nuclear antigen. However, as reverse transcription-polymerase chain reaction experiments show, these changes in protein levels are not due to the up-regulated expression of corresponding mRNAs [69]. Changes induced by cytotoxicity happen rapidly, do not last long, and do not indicate a true transdifferentiation of MSCs. A more reliable approach to induce neuronal differentiation by MSCs involves the use of morphogens and/or neurotrophic factors. When MSCs were treated by epidermal growth factor, retinoic acid (RA), or a combination of RA and BDNF, they differentiated into neuronal cells expressing markers such as nestin and NeuN [70]. Using similar induction regimens, independent studies reported the neuronal differentiation potential of MSCs both in vitro and in vivo. A number of studies have shown evidence for action potentials being fired by MSC-derived neuron-like cells [71,72]. It was hypothesized that the increase of intracellular cAMP concentration may be a key factor responsible for this transdifferentiation of MSCs [73]. Our group was the first to report that a combination of neurotrophin-3 (NT-3) and RA could induce the differentiation of MSCs into neuron-like cells [74]. Furthermore, the proportion of neuron-like cells was greatly increased when NT-3 expressing modified Schwann cells were cocultured with MSCs that were genetically modified to express tyrosine kinase C (TrkC), the receptor for NT-3 [75]. By tissue engineering, we have constructed MSC-derived neural networks on 3-dimensional (3D) scaffolds. Neuron-like cells in this environment exhibit characteristic electrophysiological behaviors, including the firing of action potentials, and postsynaptic currents [76,77]. Furthermore, MSC-derived neuron-like cells formed on these scaffolds retained neuronal phenotypes and integrated into host neural circuits with synapse-like connections. More importantly, transplantation of MSC-derived neural network tissue into the injured spinal cord significantly improved motor function in the paralyzed limbs both in a rat and a canine model. We have ruled out the possibility of the morphological changes being cytotoxicity induced, as MSC-derived neurons survived for prolonged periods in culture (up to 14 days) and in vivo (up to 6 months) [76,77]. It has been argued that neuronal differentiation of MSCs is the result of cell fusion with host neurons, resulting in a neuronal phenotype of the fused cells [78]. However, using karyotyping, we excluded this possibility [76]. Nonetheless, MSC-derived neuronal cells do not possess all features of genuine neurons. The structure of synapses formed between MSC-derived neuronal cells or between MSC-derived neuronal cells and host neurons did show some of the characteristic features of a chemical synapse, when studied by electron microscopy. Thus, we prefer to call MSC-derived neuronal cells MSC-derived neuron-like cells, based on their functional similarity to neurons, rather than morphological reasons.
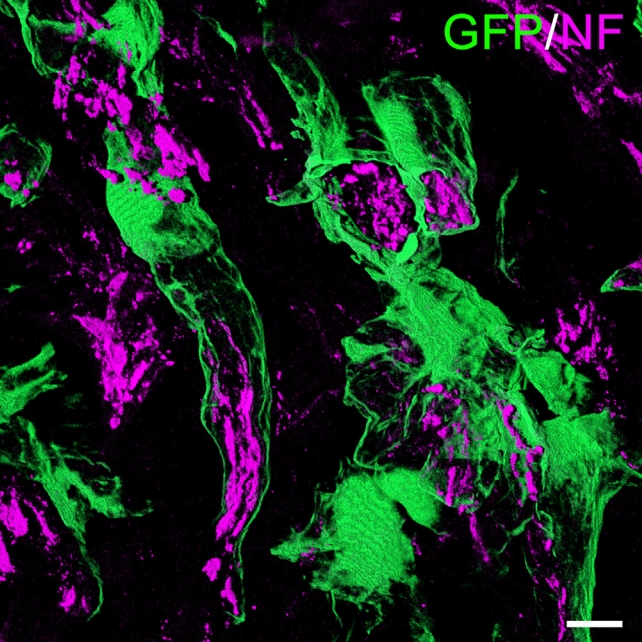
Our group recently identified a novel function of MSCs in treating SCI, the formation of perineurium-like sheaths protecting nerve fibers [8]. MSCs used in these experiments were derived from transgenic rats expressing green fluorescent protein (GFP). These cells were grafted into the transected spinal cord tissue of allogeneic rats, using a biocompatible scaffold. With the use of immunosuppression, the transplanted MSCs survived for up to 8 weeks and formed tube-like structures wrapped around nerve fibers at the injured site (Fig. 1). When examined by electron microscopy, nerve fibers covered by these tubes showed more intact morphology and contained less 4-hydroxynonenal and Nitrotyrosine. These molecules are key indicators of lipid peroxidation and protein nitration in an inflammatory microenvironment. MSCs that formed perineurium-like sheaths around neurons expressed BDNF, HGF, VEGF, glial cell line-derived neurotrophic factor (GDNF), and SOD3, a secreted antioxidant enzyme [79]. The latter indicates that perineurium-like MCS sheaths may protect nerve fibers from oxidative damage. Such MSC-derived perineurium-like sheaths also provide a physical barrier, isolating the enclosed nerve fibers from the deleterious microenvironment surrounding the injury. We performed a retrospective analysis of previously published studies transplanting MSCs to treat SCI, where donor MSCs survived beyond 3 weeks in the host spinal cord. The perineurium-like sheath structures were invariably visible on microscopic images, but were never identified or commented on by the original authors [80-87]. These findings indicate that the formation of perineurium-like sheaths by transplanted MSCs is not a fortuitous phenomenon. As discussed earlier, the microenvironment surrounding the injured cord causes excitotoxicity, oxidative damage, and is affected by deleterious immune responses. The formation of a perineurium-like sheath by MSCs can provide protection against these, aiding the regeneration of nerve fibers.
A number of clinical trials using MSCs from autologous (mostly bone marrow-derived) or allogenic (mostly umbilical cord-derived) sources have been carried out on patients with SCI [88-90]. The safety of MSCs transplantation in humans was first demonstrated in 1995. In the initial studies, adherent stromal cells isolated and cultured from bone marrow samples of patients suffering from hematological malignancies were transfused back to the donors. This early demonstration of the safety of MSCs led to an increase in the number of translational studies and clinical applications of these cells [20]. To date, dozens of registered clinical trials have been initiated to investigate the MSCs-based treatment of SCI around the world, and this number is continuously growing. Despite some encouraging observations, the fate of donor MSCs inside the human body remains largely unknown due to the absence of biopsy data, the limited number of postmortem studies, and lack of relevant imaging. Animal studies suggest that these cells only survive for a short period within the spinal cord, typically less than 2 weeks [91]. In a clinical study, autologous bone marrow-derived MSCs were labeled with superparamagnetic iron oxide nanoparticles and injected intrathecally to treat a single SCI patient. Magnetic resonance imaging detected the focal accumulation of signal 48 hours after administration. This signal faded 2 weeks later and disappeared completely 1 month after transplantation [92]. Despite their low immunogenicity, transplanted MSCs fail to persist long-term in vivo without any immunosuppression, and are thought to provide therapeutic benefits via a “Touch-and-Go”/“Hit-and-Run” mechanism [42,93 94]. However, with most of the conducted clinical studies being phase I, I/II, or II trials, the therapeutic efficacy of MSCs still needs further investigation [95]. A phase III clinical trial showed limited efficacy of a single dose of autologous MSCs in treating chronic SCI [96]. However, the administration of multiple doses of MSCs was effective in a previous study during long-term observation [89], suggesting that the therapeutic efficacy of MSCs may be dose-dependent. Thus, any intervention that increases the number of donor MSCs or promotes their survival, may produce better clinical outcomes. However, it appears that achieving this will remain a technical challenge in the treatment of SCI. There are several factors affecting therapeutic efficacy: (1) Heterogeneity of MSCs from different tissues: MSCs from the umbilical cord matrix, adipose tissue, or bone marrow show differences in their ability to inhibit peripheral blood B cells, T cells, and NK cells [97]. There are similar differences in the differentiation and proliferation of MSCs derived from different tissues [98,99]. (2) Differences in passage numbers: MSCs lose their stem cell characteristics and their telomeres get gradually shorter as the number of in vitro passages increases [100]. Although the use of cells from an early passage is recommended for clinical use, it is difficult to control for changes in this parameter when comparing trial data. (3) Differences in cell preparation processes: The lack of reproducibility in producing the transplanted MSCs in vitro may also lead to different results [101]. (4) Different routes of administration: Currently, MSCs are administered via intrathecal injection, intravenous dosing, intramuscular injection, injection in situ, or being delivered using scaffolds [102]. Intrathecal, intravenous, and intramuscular administration is less invasive but relies on the homing effect of MSCs [103]. According to animal studies, intrathecal injections appear more effective than intravenous administration [104]. Injecting MSCs directly into the spinal cord contusion cavity seems beneficial for the resolution of the glial scars and bridging axon regeneration. Thus, in situ injection to the intramedullary injury area may also achieve better outcomes [105]. More work is certainly needed to establish the optimal route of administration. (5) Different dose ranges: In various trials, the frequency of administration and the injected number of MSCs varied widely. Cell doses range from 0.5×106 to 10×106/kg, or even higher were tried using single or multiple dosage regimes [106]. (6) Individual differences: Notable differences were observed in the response of individual patients. Some appear to show significant improvements while others do not [107]. These differences could be due to several poorly quantifiable factors, including the differing extent and location of the damage, the patient’s age, prior physical condition, and the presence or absence of underlying diseases. Currently, reports on completed phase III clinical trials are scarce. Although weaknesses in study design have triggered criticism and debates [108-110], the efficacy of MSCs in the treatment of SCI demonstrated in a trial led by Stemirac is promising. In summary, although the safety of MSCs in clinical has been verified, their efficacy in treating SCI remains controversial. Data available from current clinical trials are clearly inadequate to draw final conclusions from. Much more effort will be needed before the large-scale application of MSCs for the treatment of SCI will become reality.
The fact that MSCs can target multiple pathological changes associated with the secondary injury after SCI promises tantalizing benefits. Although the efficacy of MSCs for the treatment of SCI patients is still being studied, several strategies are being developed to enhance their effectiveness in pre-clinical studies. This work may produce new MSC-derived products with improved therapeutic characteristics.
IFN-γ pretreatment could promote the secretion of soluble factors responsible for the immunosuppressive and immunomodulatory effects of the transplanted cells [106,111]. Hypoxic preconditioning followed by reoxygenation for 30 minutes improved the ability of MSCs to proliferate and migrate [112]. Research showed that culturing the cells with the oxygen level reduced to 1% could significantly increase their survival and angiogenic capacity while reducing their sensitivity to the ischemic microenvironment of the damaged spinal cord. These effects were achieved without changing the biological behavior, immunophenotype or karyotype of MSCs [113]. Transplantation of hypoxia preconditioned MSCs enhanced their protective effect during spinal cord ischemia/reperfusion injury [114]. It appears that these therapeutic benefits are due to the up-regulation of the secretion of cytokines, such as HGF and VEGF [115]. In other experiments, preconditioning with sevoflurane, an inhaled anesthetic, improved the survival and therapeutic potential of MSCs during serum deprivation and hypoxia. This was mediated through the up-regulation of HIF-1α, HIF-2α, VEGF, and p-Akt/Akt attenuating the initiation of apoptosis and the loss of mitochondrial membrane potential [115]. Other small molecules, such as IFN-γ, IL-1β, and lipopolysaccharide have also been reported to enhance the immunomodulatory properties of MSCs, causing prominent PGE-2 secretion [116].
Biomaterial scaffolds can provide a 3D milieu for embedded cells, conferring enhanced biological function during SCI repair. The physical properties of such scaffolds may alter the functional status of the seeded MSCs. The stiffness of the biomaterials can affect the morphology, proliferation, and differentiation of MSCs. MSCs cultured in alginate hydrogels of varying stiffness showed changes in gene expression profiles. The production of inflammatory regulators, including IDO1 and PGE-2, increased in stiffer matrices [117]. Other features, such as surface characteristics, or the pore size fundamentally changed the biological function of MSCs [118,119]. Furthermore, chemical modification of the scaffold may introduce new features. A hydrogel scaffold modified with the bioactive peptide PPFLMLLKGSTR significantly improved the survival and adhesive growth of MSCs in 3D cultures in vitro. This translated into better hindlimb motor function in the treated animals [120]. Coculturing MSCs with immune cells in the 3D matrix also affected their immunomodulatory potential [121]. MSCs being cultured in a polystyrene scaffold produced more anti-inflammatory cytokines, such as PGE-2 and tumor necrosis factor-stimulated gene 6. At the same time the secretion of proinflammatory cytokines, such as IL-6, monocyte chemotactic protein-1, macrophage colony-stimulating factor and receptor activator of nuclear factor κ-B ligand were reduced in cocultures with macrophages [122].
Genetic modification of MSCs could introduce new features that are useful for therapeutic purposes [123]. Transplanted MSCs genetically modified to produce insulin-like growth factor-1 have shown better survival with enhanced immunoregulation. This promoted myelination, leading to significant functional improvement after SCI [124]. MSCs modified to produce VEGF and GDNF improved angiogenesis in the injured area. This, in turn, increased the survival of transplanted cells and the extent of axonal regeneration in a rat model [125]. MSCs transduced with the BDNF gene protected the spinal cord tissue more, inhibited glial scar formation, and alleviated inflammatory responses [126]. Grafting spheroids formed by MSCs expressing BDNF promoted the retention of myelinated axons in the area of SCI and led to a significantly enhanced recovery of hindlimb motor function in a mice model [127]. Our group cocultured NT-3 expressing Schwann cells and MSCs expressing its receptor, TrkC, in a gelatin sponge scaffold. The constructed MSC-derived neural network tissue was used to repair SCI in rat and canine models [76,77]. We found that combining tissue engineering with the genetic modification of MSCs improved neural differentiation and helped the functional recovery of animals. However, the use of vectors necessary for genetic modifications, such as modified adenovirus, lentivirus, and adeno-associated virus particles, causes concerns about the safety of such genetically modified MSCs in human trials. Thus, although promising, the safety of genetically modified MSCs should be fully investigated to reduce potential harm. If their safety could be guaranteed, genetic modifications of MSCs may enhance their therapeutic utility in the treatment of SCI.
MSCs transplantation has been combined with various forms of neurorehabilitation therapy. There are reports that combining MSC transplantation with treadmill training [128], electrical stimulation [129], electroacupuncture [130], transcranial magnetic stimulation (TMS) [131], ultrashort wave therapy [132,133], and swimming training [134] resulted in improved therapeutic outcomes. Several theories were put forward to explain the increased benefits of these combined treatment regimes. (1) Tissue sparing: The combination of MSCs and TMS displayed synergistic effects on alleviating SCI-induced spinal cord lesions and neuronal apoptosis in a rat model. Increased GAP-43, NGF, and BDNF expression levels, downregulated glial fibrillary acidic protein (GFAP) expression, and reduced activation of the Raf/MEK/ERK signaling pathway was reported with this combined treatment [131]. MSCs transplantation together with physical activity such as treadmill training showed better tissue preservation, fewer microcavitations, and reduced degeneration of nerve fibers after SCI [128]. (2) Promoting donor MSCs survival: Electrical stimulation and acupuncture can promote the survival and differentiation of MSCs in rats following SCI. We have previously found that electrical acupuncture could efficiently promote the survival and differentiation of bone marrow-derived MSCs. This could lead to better axonal regeneration and locomotor recovery [130]. MSC transplantation combined with electroacupuncture therapy can also improve Basso-Beattie-Bresnahan scale scores and evoked motor potentials. The number of neurofilament-positive and Biotinylated dextran amine-labeled axons increased, leading to improved outcomes [135]. There is evidence that treadmill training improves the survival of neural precursor cells in the post-SCI microenvironment, through the involvement of MSCs [136]. (3) Modulation of neuroinflammation and reduction of glial scarring: Other experiments combined human umbilical cord MSCs with ultrashort wave therapy in SCI. This combination improved motor function, and decreased the number of infiltrating CD3+ T cells, while decreasing microglia and astrocyte inflammation [132,133]. Moreover, decreased GFAP and chondroitin sulfate proteoglycans expression was detected in this combination treatment group [135]. What is the mechanism behind the improved outcomes when MSCs transplantation is combined with neurorehabilitation? Our study showed that electroacupuncture increased the secretion of NT-3 from the injured spinal cord tissue. This, in turn, can promote the survival, differentiation, and migration of grafted MSCs expressing the TrkC gene [137]. Subsequently, we explored the mechanism that allows electroacupuncture to promote the increased secretion of NT-3 by the activation of the CGRP/RAMP1/αCaMKII pathway [138]. In a recently published review, we summarized the potential mechanisms explaining the benefits of combining electroacupuncture with MSCs transplantation in the treatment of SCI [139].
The exact mechanism by which MSCs improve outcomes after SCI remains to be explored further. This work will offer further insights into enhancing their safe and effective therapeutic use. In this review, we have shown that MSCs have multiple functional properties that can target a variety of pathological consequences of SCI (Fig. 2). One of these is a novel finding, the perineurium-like differentiation of MSCs, that warrants further investigation. Future basic research needs to be focused on developing MSC-based treatment strategies that improve the efficacy of treatment in SCI.
NOTES
Funding/Support
This research was supported by grants from the Chinese National Natural Science Foundation (Grant No. 31900975 to Y. H. Ma); the Natural Science Foundation of Guangdong Province (Grant Nos. 2018A030310110 and 2020-A1515011537 to Y. H. Ma); the Start-up Fund Projects of Guangzhou First People’s Hospital (Grant No. KYQD20210017 to Y. H. Ma) and the Startup Grant of Guangdong Hospital of Chinese Medicine (Grant No.2022KT1032) to Xiang Zeng.
Fig. 1.
Transplanted mesenchymal stem cells derived from green fluorescent protein (GFP) transgenic donor rats form perineurium-like sheaths (green) around bundles of neurofilament (NF)-positive nerve fibers (purple) at the site of the injury in a rat with transecting spinal cord injury. Bar = 10 μm.
REFERENCES
1. National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. Birmingham (AL): University of Alabama at Birmingham; 2021.
2. Takami T, Shimokawa N, Parthiban J, et al. Pharmacologic and regenerative cell therapy for spinal cord injury: WFNS Spine Committee Recommendations. Neurospine 2020;17:785-96.




3. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013;13:392-402.


4. Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. BioMed Res Int 2013;2013:786475.




5. Zeng X, Zeng YS, Ma YH, et al. Bone marrow mesenchymal stem cells in a three-dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis, and reduce cavity formation in experimental spinal cord injury. Cell Transplant 2011;20:1881-99.



6. Yang Y, Cao TT, Tian ZM, et al. Subarachnoid transplantation of human umbilical cord mesenchymal stem cell in rodent model with subacute incomplete spinal cord injury: preclinical safety and efficacy study. Exp Cell Res 2020;395:112184.


7. Cao TT, Chen H, Pang M, et al. Dose optimization of intrathecal administration of human umbilical cord mesenchymal stem cells for the treatment of subacute incomplete spinal cord injury. Neural Regen Res 2022;17:1785-94.



8. Ma YH, Zeng X, Qiu XC, et al. Perineurium-like sheath derived from long-term surviving mesenchymal stem cells confers nerve protection to the injured spinal cord. Biomaterials 2018;160:37-55.


9. Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol 2019;10:282.



10. Badhiwala JH, Ahuja CS, Fehlings MG. Time is spine: a review of translational advances in spinal cord injury. Journal of neurosurgery. Spine 2018;30:1-18.

11. Neirinckx V, Cantinieaux D, Coste C, et al. Concise review: Spinal cord injuries: how could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells 2014;32:829-43.



12. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 1970;3:393-403.


13. Beresford JN, Bennett JH, Devlin C, et al. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 1992;102(Pt 2):341-51.



14. Mardon HJ, Bee J, von der Mark K, et al. Development of osteogenic tissue in diffusion chambers from early precursor cells in bone marrow of adult rats. Cell Tissue Res 1987;250:157-65.



15. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-7.


17. Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 2015;16:239-53.


18. Lee MW, Yang MS, Park JS, et al. Isolation of mesenchymal stem cells from cryopreserved human umbilical cord blood. Int J Hematol 2005;81:126-30.


19. Schu S, Nosov M, O'Flynn L, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med 2012;16:2094-103.



20. Wang Y, Han ZB, Song YP, et al. Safety of mesenchymal stem cells for clinical application. Stem Cells Int 2012;2012:652034.




21. Teng YD, Yu D, Ropper AE, et al. Functional multipotency of stem cells: a conceptual review of neurotrophic factorbased evidence and its role in translational research. Curr Neuropharmacol 2011;9:574-85.



22. Diez Villanueva P, Sanz-Ruiz R, Nunez Garcia A, et al. Functional multipotency of stem cells: what do we need from them in the heart? Stem Cells Int 2012;2012:817364.


23. Ropper AE, Thakor DK, Han I, et al. Defining recovery neurobiology of injured spinal cord by synthetic matrixassisted hMSC implantation. Proc Natl Acad Sci U S A 2017;114:E820-9.



24. Tao H, Han Z, Han ZC, et al. Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int 2016;2016:1314709.




25. King A, Balaji S, Keswani SG, et al. The role of stem cells in wound angiogenesis. Adv Wound Care (New Rochelle) 2014;3:614-25.



26. Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: a review. World J Stem Cells 2014;6:120-33.



27. Zanotti L, Angioni R, Cali B, et al. Mouse mesenchymal stem cells inhibit high endothelial cell activation and lymphocyte homing to lymph nodes by releasing TIMP-1. Leukemia 2016;30:1143-54.




28. De Luca A, Gallo M, Aldinucci D, et al. Role of the EGFR ligand/receptor system in the secretion of angiogenic factors in mesenchymal stem cells. J Cell Physiol 2011;226:2131-8.


29. Zhao Q, Ren H, Han Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J Cell Immunother 2016;2:3-20.

30. Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:11-20.


31. Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838-43.



32. Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003;75:389-97.


33. Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42-8.


34. Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003;102:3837-44.



35. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22.



36. Yan Z, Zhuansun Y, Chen R, et al. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res 2014;324:65-74.


37. Chen K, Wang D, Du WT, et al. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol 2010;135:448-58.


38. Xu C, Yu P, Han X, et al. TGF-beta promotes immune responses in the presence of mesenchymal stem cells. J Immunol 2014;192:103-9.

39. Ling W, Zhang J, Yuan Z, et al. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res 2014;74:1576-87.




40. Yen BL, Yen ML, Hsu PJ, et al. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Reports 2013;1:139-51.



41. Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006;107:367-72.



42. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726-36.



43. Rosado MM, Bernardo ME, Scarsella M, et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev 2015;24:93-103.



44. Fan L, Hu C, Chen J, et al. Interaction between mesenchymal stem cells and B-cells. Int J Mol Sci 2016;17:650.



45. Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 2008;26:151-62.



46. Cassatella MA, Mosna F, Micheletti A, et al. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells 2011;29:1001-11.



47. Gao S, Mao F, Zhang B, et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-kappaB and signal transducer and activator of transcription 3 pathways. Exp Biol Med (Maywood) 2014;239:366-75.



48. Scuteri A, Miloso M, Foudah D, et al. Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr Stem Cell Res Ther 2011;6:82-92.


49. Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther 2016;7:131.




50. Liudmila N, Novikova MB, Paul J, et al. Neuroprotective and growth-promoting effects of bone marrow stromal cells after cervical spinal cord injury in adult rats. Cytotherapy 2011;13:873-87.


51. Chung HJ, Chung WH, Lee JH, et al. Expression of neurotrophic factors in injured spinal cord after transplantation of human-umbilical cord blood stem cells in rats. J Vet Sci 2016;17:97-102.



52. Menezes K, Nascimento MA, Goncalves JP, et al. Human mesenchymal cells from adipose tissue deposit laminin and promote regeneration of injured spinal cord in rats. PLoS One 2014;9:e96020.



53. Zeng X, Ma YH, Chen YF, et al. Autocrine fibronectin from differentiating mesenchymal stem cells induces the neurite elongation in vitro and promotes nerve fiber regeneration in transected spinal cord injury. J Biomed Mater Res A 2016;104:1902-11.




54. Guo S, Redenski I, Levenberg S. Spinal cord repair: from cells and tissue engineering to extracellular vesicles. Cells 2021;10:1872.



55. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 2016;428:688-92.



56. Buschow SI, Nolte-'t Hoen EN, van Niel G, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 2009;10:1528-42.


57. Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J 2007;26:4263-72.



58. Hosseini Shamili F, Alibolandi M, Rafatpanah H, et al. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Control Release 2019;299:149-64.


59. Dong R, Liu Y, Yang Y, et al. MSC-derived exosomes-based therapy for peripheral nerve injury: a novel therapeutic strategy. BioMed Res Int 2019;2019:6458237.




60. Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci 2014;15:4142-57.



61. Koga Y, Yasunaga M, Moriya Y, et al. Exosome can prevent RNase from degrading microRNA in feces. J Gastrointest Oncol 2011;2:215-22.


62. Katsuda T, Kosaka N, Takeshita F, et al. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 2013;13:1637-53.



63. Liu W, Wang Y, Gong F, et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of a1 neurotoxic reactive astrocytes. J Neurotrauma 2019;36:469-84.


64. Lankford KL, Arroyo EJ, Nazimek K, et al. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One 2018;13:e0190358.



65. Huang JH, Yin XM, Xu Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J Neurotrauma 2017;34:3388-96.


66. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7.


67. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells 2007;25:2896-902.



68. Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 2000;61:364-70.


69. Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res 2004;77:174-91.


70. Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 2000;164:247-56.


71. Krabbe C, Zimmer J, Meyer M. Neural transdifferentiation of mesenchymal stem cells--a critical review. APMIS 2005;113:831-44.


72. Barzilay R, Melamed E, Offen D. Introducing transcription factors to multipotent mesenchymal stem cells: making transdifferentiation possible. Stem Cells 2009;27:2509-15.



73. Deng W, Obrocka M, Fischer I, et al. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun 2001;282:148-52.


74. Zhang W, Zeng YS, Zhang XB, et al. Combination of adenoviral vector-mediated neurotrophin-3 gene transfer and retinoic acid promotes adult bone marrow cells to differentiate into neuronal phenotypes. Neurosci Lett 2006;408:98-103.


75. Zhang YQ, Zeng X, He LM, et al. NT-3 gene modified Schwann cells promote TrkC gene modified mesenchymal stem cells to differentiate into neuron-like cells in vitro. Anat Sci Int 2010;85:61-7.



76. Zeng X, Qiu XC, Ma YH, et al. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials 2015;53:184-201.


77. Wu GH, Shi HJ, Che MT, et al. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials 2018;181:15-34.


78. Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 2003;425:968-73.



79. Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 2002;33:337-49.


80. Parr AM, Kulbatski I, Wang XH, et al. Fate of transplanted adult neural stem/progenitor cells and bone marrow-derived mesenchymal stromal cells in the injured adult rat spinal cord and impact on functional recovery. Surg Neurol 2008;70:600-7. discussion 7.


81. Hyatt AJ, Wang D, van Oterendorp C, et al. Mesenchymal stromal cells integrate and form longitudinally-aligned layers when delivered to injured spinal cord via a novel fibrin scaffold. Neurosci Lett 2014;569:12-7.



82. Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurology 2005;191:344-60.

83. Novikova LN, Brohlin M, Kingham PJ, et al. Neuroprotective and growth-promoting effects of bone marrow stromal cells after cervical spinal cord injury in adult rats. Cytotherapy 2011;13:873-87.


84. Pastor D, Viso-Leon MC, Jones J, et al. Comparative effects between bone marrow and mesenchymal stem cell transplantation in GDNF expression and motor function recovery in a motorneuron degenerative mouse model. Stem Cell Rev Rep 2012;8:445-58.



85. Sandner B, Ciatipis M, Motsch M, et al. Limited functional effects of subacute syngeneic bone marrow stromal cell transplantation after rat spinal cord contusion injury. Cell Transplant 2016;25:125-39.



86. Sheth RN, Manzano G, Li X, et al. Transplantation of human bone marrow-derived stromal cells into the contused spinal cord of nude rats. Journal of neurosurgery. Spine 2008;8:153-62.

87. Torres-Espin A, Redondo-Castro E, Hernandez J, et al. Immunosuppression of allogenic mesenchymal stem cells transplantation after spinal cord injury improves graft survival and beneficial outcomes. J Neurotrauma 2015;32:367-80.



88. Sykova E, Homola A, Mazanec R, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant 2006;15:675-87.



89. Park JH, Kim DY, Sung IY, et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery 2012;70:1238-47. discussion 47.



90. Mendonca MV, Larocca TF, de Freitas Souza BS, et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther 2014;5:126.




91. Brock JH, Graham L, Staufenberg E, et al. Bone marrow stromal cell intraspinal transplants fail to improve motor outcomes in a severe model of spinal cord injury. J Neurotrauma 2016;33:1103-14.



92. Chotivichit A, Ruangchainikom M, Chiewvit P, et al. Chronic spinal cord injury treated with transplanted autologous bone marrow-derived mesenchymal stem cells tracked by magnetic resonance imaging: a case report. J Med Case Rep 2015;9:79.




93. Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 2008;103:1204-19.



94. von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012;30:1575-8.



95. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 2016;25:829-48.



96. Oh SK, Choi KH, Yoo JY, et al. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery 2016;78:436-47. discussion 47.



97. Ribeiro A, Laranjeira P, Mendes S, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther 2013;4:125.




98. in’t Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 2003;88:845-52.

99. Hass R, Kasper C, Bohm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011;9:12.




100. Bonab MM, Alimoghaddam K, Talebian F, et al. Aging of mesenchymal stem cell in vitro. BMC Cell Biol 2006;7:14.




101. Robb KP, Fitzgerald JC, Barry F, et al. Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy 2019;21:289-306.


102. Vismara I, Papa S, Rossi F, et al. Current options for cell therapy in spinal cord injury. Trends Mol Med 2017;23:831-49.


103. Cofano F, Boido M, Monticelli M, et al. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci 2019;20.

104. Paul C, Samdani AF, Betz RR, et al. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine 2009;34:328-34.



105. Oh SK, Jeon SR. Current concept of stem cell therapy for spinal cord injury: a review. Korean J Neurotrauma 2016;12:40-6.




106. Gao F, Chiu SM, Motan DA, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 2016;7:e2062.




107. Bydon M, Dietz AB, Goncalves S, et al. CELLTOP clinical trial: first report from a phase 1 trial of autologous adipose tissue-derived mesenchymal stem cells in the treatment of paralysis due to traumatic spinal cord injury. Mayo Clin Proc 2020;95:406-14.


109. Cyranoski D. Japan's approval of stem-cell treatment for spinal-cord injury concerns scientists. Nature 2019;565:544-5.



111. DelaRosa O, Lombardo E, Beraza A, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A 2009;15:2795-806.

112. Kheirandish M, Gavgani SP, Samiee S. The effect of hypoxia preconditioning on the neural and stemness genes expression profiling in human umbilical cord blood mesenchymal stem cells. Transfus Apher Sci 2017;56:392-9.


113. Bader AM, Klose K, Bieback K, et al. Hypoxic preconditioning increases survival and pro-angiogenic capacity of human cord blood mesenchymal stromal cells in vitro. PLoS One 2015;10:e0138477.



114. Wang Z, Fang B, Tan Z, et al. Hypoxic preconditioning increases the protective effect of bone marrow mesenchymal stem cells on spinal cord ischemia/reperfusion injury. Mol Med Rep 2016;13:1953-60.



115. Sun X, Fang B, Zhao X, et al. Preconditioning of mesenchymal stem cells by sevoflurane to improve their therapeutic potential. PLoS One 2014;9:e90667.



116. Saparov A, Ogay V, Nurgozhin T, et al. Preconditioning of human mesenchymal stem cells to enhance their regulation of the immune response. Stem Cells Int 2016;2016:3924858.




117. Darnell M, Gu L, Mooney D. RNA-seq reveals diverse effects of substrate stiffness on mesenchymal stem cells. Biomaterials 2018;181:182-8.



118. Zhu Y, Zhang K, Zhao R, et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials 2017;147:133-44.


119. Yuan T, Li K, Guo L, et al. Modulation of immunological properties of allogeneic mesenchymal stem cells by collagen scaffolds in cartilage tissue engineering. J Biomed Mater Res A 2011;98:332-41.


120. Li LM, Han M, Jiang XC, et al. Peptide-tethered hydrogel scaffold promotes recovery from spinal cord transection via synergism with mesenchymal stem cells. ACS Appl Mater Interfaces 2017;9:3330-42.

121. Chen Y, Shu Z, Qian K, et al. Harnessing the properties of biomaterial to enhance the immunomodulation of mesenchymal stem cells. Tissue Eng Part B Rev 2019;25:492-9.


122. Valles G, Bensiamar F, Crespo L, et al. Topographical cues regulate the crosstalk between MSCs and macrophages. Biomaterials 2015;37:124-33.



123. Wei W, Huang Y, Li D, et al. Improved therapeutic potential of MSCs by genetic modification. Gene Ther 2018;25:538-47.



124. Allahdadi KJ, de Santana TA, Santos GC, et al. IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury. Stem Cell Res Ther 2019;10:146.




125. Mukhamedshina YO, Garanina EE, Masgutova GA, et al. Assessment of glial scar, tissue sparing, behavioral recovery and axonal regeneration following acute transplantation of genetically modified human umbilical cord blood cells in a rat model of spinal cord contusion. PLoS One 2016;11:e0151745.



126. Li LM, Huang LL, Jiang XC, et al. Transplantation of BDNF gene recombinant mesenchymal stem cells and adhesive peptide-modified hydrogel scaffold for spinal cord repair. Curr Gene Ther 2018;18:29-39.


127. Uchida S, Hayakawa K, Ogata T, et al. Treatment of spinal cord injury by an advanced cell transplantation technology using brain-derived neurotrophic factor-transfected mesenchymal stem cell spheroids. Biomaterials 2016;109:1-11.


128. Massoto TB, Santos ACR, Ramalho BS, et al. Mesenchymal stem cells and treadmill training enhance function and promote tissue preservation after spinal cord injury. Brain Res 2020;1726:146494.


129. Krueger E, Magri LMS, Botelho AS, et al. Effects of low-intensity electrical stimulation and adipose derived stem cells transplantation on the time-domain analysis-based electromyographic signals in dogs with SCI. Neurosci Lett 2019;696:38-45.


130. Ding Y, Yan Q, Ruan JW, et al. Electro-acupuncture promotes survival, differentiation of the bone marrow mesenchymal stem cells as well as functional recovery in the spinal cord-transected rats. BMC Neurosci 2009;10:35.




131. Feng S, Wang S, Sun S, et al. Effects of combination treatment with transcranial magnetic stimulation and bone marrow mesenchymal stem cell transplantation or Raf inhibition on spinal cord injury in rats. Mol Med Rep 2021;23:294.



132. Wang S, Jia Y, Cao X, et al. HUCMSCs transplantation combined with ultrashort wave therapy attenuates neuroinflammation in spinal cord injury through NUR77/NF-kappaB pathway. Life Sci 2021;267:118958.

133. Na L, Wang S, Liu T, et al. Ultrashort wave combined with human umbilical cord mesenchymal stem cell (HUC-MSC) transplantation inhibits NLRP3 inflammasome and improves spinal cord injury via MK2/TTP signalling pathway. BioMed Res Int 2020;2020:3021750.




134. Carvalho KA, Cunha RC, Vialle EN, et al. Functional outcome of bone marrow stem cells (CD45(+)/CD34(-)) after cell therapy in acute spinal cord injury: in exercise training and in sedentary rats. Transplant Proc 2008;40:847-9.


135. Ding Y, Yan Q, Ruan JW, et al. Bone marrow mesenchymal stem cells and electroacupuncture downregulate the inhibitor molecules and promote the axonal regeneration in the transected spinal cord of rats. Cell Transplant 2011;20:475-91.



136. Younsi A, Zheng G, Scherer M, et al. Treadmill training improves survival and differentiation of transplanted neural precursor cells after cervical spinal cord injury. Stem Cell Res 2020;45:101812.


137. Yang Y, Xu HY, Deng QW, et al. Electroacupuncture facilitates the integration of a grafted TrkC-modified mesenchymal stem cell-derived neural network into transected spinal cord in rats via increasing neurotrophin-3. CNS Neurosci Ther 2021;27:776-91.




138. Xu H, Yang Y, Deng QW, et al. Governor vessel electroacupuncture promotes the intrinsic growth ability of spinal neurons through activating calcitonin gene-related peptide/alpha-calcium/calmodulin-dependent protein kinase/neurotrophin-3 pathway after spinal cord injury. J Neurotrauma 2021;38:734-45.


- TOOLS