- Search
|
|
||
Abstract
Symptomatic thoracic disc herniations are a rare entity and their operative treatment is challenging. Open approaches, despite providing excellent access, are associated with significant access morbidity from thoracotomy, and this has led to an increased interest in minimally invasive techniques such as mini-open approach, thoracoscopic approach and the endoscopic approach. In this article, we describe the technical points for performing a transforaminal endoscopic thoracic discectomy and summarize its literature outcomes in the context of other minimally invasive approaches.
Symptomatic thoracic disc herniations are a relatively rare entity in that they represent less than 1% of all disc herniations, however, literature from autopsy and magnetic resonance imaging (MRI) studies has reported an incidence of up to 15.2% of thoracic disc herniations in the general population [1-7]. Although many maybe asymptomatic, clinical presentation of thoracic disc herniations can be quite significant and include myelopathy, thoracic radiculopathy, axial back pain, gait instability and bowel and/or bladder dysfunction [2].
The operative treatment of thoracic disc herniations is challenging due to the presence of ribs, narrow spinal canal dimensions and vascular anatomy of the spinal cord. Initial approaches utilizing a central decompressive laminectomy were fraught with iatrogenic complications due to the manipulation of the spinal cord, which paved the way for other approaches including transpedicular and costotransversectomy [2,8]. However, the limited working window of the aforementioned approaches led to the widespread adoption of the anterior transthoracic approach which utilizes a thoracotomy to perform the discectomy, followed by potential fusion based on the amount of bony resection [2,8].
Despite providing excellent access, the associated morbidity of a thoracotomy including postthoracotomy pain led to an increased interest in minimally invasive techniques for approaching thoracic disc herniations such as mini-open approach, thoracoscopic approach and the transforaminal endoscopic approach [7,9]. The transforaminal approach was first reported by Kambin who described the safe transforaminal triangle for a percutaneous discectomy in 1973 [10]. After instrumentation and technique improvements and popularization of its use in the lumbar spine, the past decade witnessed an increased use of the transforaminal endoscopic approach in the thoracic spine [11,12].
We herein describe the technical points of performing a transforaminal endoscopic thoracic discectomy (TETD) and summarize the outcomes in the literature.
Any thoracic disc herniations at any level, except T1/2 could be a candidate of TETD. T1/2 foraminal or paracentral disc herniations can be accessed with a posterior interlaminar approach like a posterior cervical endoscopic foraminotomy and discectomy, given the risk of functioning T1 nerve root damage and narrow intercostal space.
Paracentral soft disc herniation with myelopathy with/without thoracic radiculopathy will be the ideal indication of TETD (Fig. 1). Since not a small number of thoracic disc herniations show calcification, partially calcified thoracic disc herniations would also be an indication of TETD, unless it is an extensive ossification of the posterior longitudinal ligament. If the calcified disc herniation has a dural adhesion, a ‘floating’ technique; involving the removal of the disc and leaving the calcific shell if the calcified disc is all detached from the disc and the posterior longitudinal ligament, may provide significant improvement (Fig. 2). Combined ossification of the yellow ligament (ossification of ligamentum flavum) may not be effective with TETD, but cases combined with central/paracentral disc herniation could have favorable outcomes as enucleation through TETD can provide some central decompression after shrinkage of the disc material.
Central disc herniations can be more difficult to access than paracentral disc herniations. The central disc fragment can be removed successfully with TETD approach if it is a loose soft nucleus fragment, through a more shallow access angle after the transforaminal approach. But effective central decompression may not be easy when the central disc is calcified or has a thickened annulus component. In those cases, central enucleation could cause shrinkage of the central fragment with the healing process, but this is not always possible.
The patient is positioned prone over a radiolucent operative table. Intraoperative neuromonitoring including somatosensory evoked potential and motor evoked potential are optional if the procedure is under general anesthesia. The authors recommend neuro-monitoring when spinal cord compression is severe or when the patient has underlying significant neurological deficits. At the authors’ institution, neuromonitoring is routinely performed for surgery involving any spinal cord level because of possible medicolegal issues if it is not contraindicated.
The arms are positioned on side arm boards and the leg position can be in mild flexion of hip and knee joints without any pressure points. The thoracic spine area is prepped and draped in a sterile fashion and a C-arm is positioned so that true anteroposterior (AP), lateral and oblique views are available. Irrigation pump pressure is set at 35 mmHg with room temperature saline.
The portal for TETD would be variable depending on the pathology; paracentral versus central disc herniation. A central disc herniation may need a portal with more lateral location. Also, the soft tissue thickness of the patient at the level of access is another factor to consider. The distance from the midline varies from 5 cm to 9 cm. The easiest way for localization is drawing a line from the target point to the skin and measuring the distance between the midline and the skin entry on a computed tomography or MRI gantry axial image (Fig. 3).
Generally, the access trajectory targets the lateral aspect of the facet for additional lateral facetectomy and foraminal expansion (foraminoplasty). Usually, the access angle is steeper (smaller angle from the midline) than conventional lumbar transforaminal discectomy, around 45°, because of the convexity of the rib cage and pleural cavity. But an operator may lower their hand to increase the approach angle (increase the angle from midline) with the same portal after decompression of the paracentral area, to gain better access to the central spinal canal.
Under fluoroscopic guidance, an 8-inch discography needle is placed targeting the posterolateral corner of the disc. The end of needle should be located at the center to the lateral half of the lower pedicle on an AP view and posterior aspect of the disc on a lateral view for a good starting point (Fig. 4). Then the needle can be advanced toward the center of the disc to inject diluted methylene blue to stain the disc material as needed. Sequential soft tissue dilators and obturator are inserted before the placement of the working sleeve (Fig. 4).
Intervertebral foramen of the upper to mid thoracic spine, from T2 to T10, have different anatomical characteristics compared to the lower thoracic spine from T10–12.
The intervertebral foramina have smaller dimensions, the disc spaces are partially covered by the corresponding rib head at T9/10 and above. Lateral facetectomy and rib head resection are required to access the epidural space at the upper and mid thoracic spine. Transforaminal approach to T10/11, T11/12 and T12/L1 disc space will be similar to the upper lumbar spine, because their intervertebral foramina are wider, and the disc spaces are not covered by the rib heads (Fig. 5).
Another unique point of the transforaminal approach is that the pedicles of thoracic spine are caudally angled compared to the disc space, as such the superior aspect of the lower pedicle is partially blocking the access of the disc space plane (Fig. 5).
After placement of the working sleeve, the soft tissue consisting of muscles and ligaments should be cleaned using a flexible radiofrequency tip to expose the lateral aspect of the facet and rib head. Using an endoscopic drill (usually 3.5-mm diameter diamond burr), the lateral aspect of the facet joint is burred to access the disc space (up to 50 % of the joint) (Fig. 6). The superomedial aspect of the rib head may require resection as needed to expose the disc space or advance the working cannula towards the midline. The rib head can be resected using an endoscopic burr. Superior pediculectomy is required if adequate visualization of the disc is not enough with lateral facetectomy alone (Fig. 6). Once the foramen has been enlarged with lateral facetectomy and the rib head resection, the next steps are similar to the endoscopic lumbar discectomy.
After docking the beveled end of working cannula on the posterolateral corner of the disc, endoscopic scalpel, cutting punches or variable sized endoscopic pituitary rongeurs can be utilized for the annulotomy and disc fragment removal (Fig. 7). Injecting 2–3 mL of diluted methylene blue in the disc space with an 8-inch discography needle will be helpful to locate the ruptured disc fragments.
Thoracic intervertebral disc spaces are usually not high enough to advance a 7-mm diameter working cannula. To access the more central side, both upper and lower end plates can be partially removed using a small diameter (2.5 or 3.5 mm) endoscopic drill. Calcified annulus can be drilled also (Fig. 7). When the ventral epidural space with epidural fat and pulsating dural sac are visible under endoscopic vision, the decompression would be sufficient (Fig. 7).
Traditionally, thoracic disc herniations are accessed through open approaches such as transpedicular, costotransversectomy or anterior transthoracic. More recently, less invasive options including mini-open, thoracoscopic and endoscopic approaches have become popular.
There have been a few case series in the literature describing TETD and its outcomes (Table 1). In 2010, Choi et al. [4] initially reported on 14 patients who underwent TETD for soft thoracic disc herniations under local anesthesia with sedation and showed improvements in patient visual analogue scale (VAS) for back and leg pain and Oswestry-Disability Index (ODI) scores. Their mean operative time was 61 minutes and there were no surgical related complications reported.
Bae et al. [5] reported their case series of 92 consecutive patients with symptomatic thoracic soft disc herniations who underwent TETD under local anesthesia with sedation. At a mean followup time of 38.4 months there was significant improvement in both VAS pain and ODI scores. Their complications included one patient with transient motor weakness, 3 patients with lower extremity parasthesias, and 2 patients with symptomatic recurrent herniation with 1 requiring reoperation and the other improving nonoperatively.
For patients with lower thoracic spinal stenosis, Guo et al. [13] reported on 6 consecutive patients who underwent transforaminal endoscopic thoracic decompression and discectomy and demonstrated significant improvement in the Japanese Orthopaedic Association scale from a mean of 4.4 preoperatively to 6.6 at 1-year follow-up, and improvement in Frankel grade of all patients from incomplete motor loss (grade D) to normal (grade E) by the 3-month follow-up mark with no reported thoracic, vascular, infectious or permanent neurologic complications.
As reported by Uribe et al. [2], open approaches for thoracic disc herniations involve a mean blood loss of 562 mL and a mean hospital length of stay (LOS) of 8.6 days. An endoscopic approach minimizes the invasiveness and provides access to the spinal cord generally without the need for a facetectomy or pedicle removal, which in some cases can prevent the need for fusion as well. Moreover, apart from the improved cosmesis, reduced risk of infection, and lower blood loss, patients who undergo endoscopic technique do not need a chest tube and can be done under local anesthesia, resulting in a quicker recovery with a shorter hospital LOS compared with open approaches [4,7].
While mini-open approaches are also becoming more popular due to their decreased invasiveness, the approach generally remains similar to the open approach in addition to the required bony work that still carries the risk of destabilizing facet bone [2,8]. Uribe reported a series of 60 patients undergoing mini-open lateral approach for thoracic disc herniations, of which 55% were calcified. Seventy-five percent underwent a transpleural approach while 25% underwent a retropleural approach. They reported a median operative time of 182 minutes, median LOS of 5 days, and median blood loss was 290 mL. Most patients showed improvements in myelopathy, radiculopathy and pain, although 10% underwent posterior supplemental fixation for instability, and 78% required a chest tube. Complications included a durotomy in 7 cases (11.7%), one each of pneumonia, extrapleural free air, new lower extremity weakness, wound infection in posterior instrumentation, and mild intercostal neuralgia. There were 3 cases (5%) requiring reoperation for re-exploration, wound infection, and residual disc removal [2].
Bae et al. [7] compared 38 patients who underwent a microscopic posterior/posterolateral approach utilizing hemilaminectomy, medial facetectomy and in some cases partial pediculotomy for thoracic disc herniations, with 39 patients who underwent a TETD for the same indication at a single institution. They demonstrated shorter operative time (70.6 minutes vs. 175.7 minutes), lower blood loss (3.8 mL vs. 357.4 mL), shorter hospital LOS (7 days vs. 13 days), and greater patient satisfaction based on MacNab criteria (89.7% vs. 73%) between the TETD and microscopic approaches, respectively. However, patients who underwent the microscopic approach had a greater proportion of concomitant ossification of ligamentum flavum than the TETD group (34.2% vs. 2.6%).
Thoracoscopic and transforaminal techniques are similar in that they are both needle-based and require the use of specialized endoscopes. Thoracoscopy however often requires a thoracic access surgeon given the lack of familiarity of working through the thoracic cavity with an endoscope, compared to the more posterior/posterolateral based transforaminal approach. In addition, TETD can be performed with local anesthesia and sedation which has increased in popularity lately in select patients to facilitate early recovery pathways [4,8,14]. Thoracoscopy on the other hand requires the use of general anesthesia and single-lung ventilation. However, general anesthesia has benefits for neuromonitoring and is more comfortable for both patients and surgeons, although the ability to check a patient’s response under local anesthesia may obviate the need for neuromonitoring [7]. Quint et al. [3] reported their prospective cohort of 167 patients who underwent single level thoracoscopic discectomy and demonstrated improvements in VAS pain scores and the American Spinal Injury Association motor score at 2-year follow-up. Their reported complications included transient intercostal neuralgia (5.4%), dural tears (1.2%), respiratory complications (3.6% - including pleuritis, pneumothorax and pleural effusions), symptomatic postoperative instability (1.8%), incomplete decompression (1.8%), and motor deficit (1.2%).
Despite its overall advantages, TETD comes with its own limitations. Similarly, to the thoracoscopic approach, the cost of instrumentation, the 2-dimensional visualization of a 3-dimensional (3D) pathology, steep learning curve and difficulty in managing intraoperative complications are real concerns [2,7]. In addition, TETD is more difficult in the upper thoracic spine due to the rib heads limiting foraminal access and the presence of the scapula [15]. Bae et al. [16] however demonstrated its feasibility in a series of 14 consecutive patients with soft disc herniations who underwent transforaminal endoscopic discectomy between T2 and T6 without any reported neurologic or vascular complications. Traditionally, relative contraindications for a transforaminal endoscopic approach are calcified or sequestered discs due to the limited working window as a result of the constraints on the position of the endoscope [4,8,17]. However, there have been reports of successful treatment of calcific thoracic discs performed endoscopically [13,18-21]. Houra et al. [18] reported their series of 16 patients treated with TETD for thoracic disc herniations, with 10 having partially or fully calcified discs, and demonstrating significant VAS and ODI improvements at 5-year follow-up and without reported surgical complications. The same author also showed that good outcomes are obtained without complications in 2 patients with large 2-level calcified disc herniations in the midlower thoracic spine using the endoscopic transforaminal approach [19]. Moreover, Gao et al. [6] reported their series of 11 patients with thoracic disc herniations, 9 of which were calcified, who TETD, without postoperative nerve injury, infection or hematoma formation.
Complications of TETD have been variably reported in the literature. Ruetten et al. [20] reported a 20% (5 of 25) rate of complications including 1 dural tear during the resection of a calcific disc herniation (was covered with a synthetic dural substitute and a fat flap), 1 epidural hematoma, 2 transient intercostal neuralgias, and 1 deterioration of myelopathy from a giant disc herniation. No infections, pulmonary complications, or instability were reported up to 18 months of follow-up. Other complications such as vascular injuries and incomplete decompression are also possible especially in cases of inexperience/unfamiliarity with the anatomy or instrumentation. Pulmonary complications including pneumothorax due to targeting needle puncturing the pleura have also been reported, with some authors modifying the technique to using dilators without a targeting needle [6]. Although there has not been a study specifically addressing the learning curve of TETD, a systematic review of complications associated with the learning curve of minimally invasive spine surgery in general demonstrated that operative time and complications are usually overcome in 20–30 consecutive cases for most minimally invasive techniques [22], and for full endoscopic lumbar discectomy, learning curves of 22–33 cases are reported in the literature [23,24].
A unilateral endoscopic interlaminar approach has also been described in the thoracic spine. Ruetten et al. [25] reported on full endoscopic decompression of the thoracic spine including 20 cases using the interlaminar approach and 26 cases using the transforaminal approach with favorable outcomes. They reported 1 case of epidural hematoma and 1 case of transient arm dysesthesia as the complications of interlaminar approach. Given the minimal retractability of the thoracic dura and spinal cord, the interlaminar approach will be limited for most paracentral disc herniations. However, the interlaminar approach would be useful in the selected cases of a disc herniation that is extruded past the lateral border of the dura or a dorsally migrated disc herniation.
NOTES
Fig. 1.
A 72-year-old female with thoracic myelopathy. (A, B) Preoperative magnetic resonance imaging show right paracentral disc extrusion with spinal cord compression (the circle and arrow) with intramedullary signal changes. (C, D) Postoperative images show improved spinal cord signal and space. The patient had significant improvement of myelopathy after surgery.

Fig. 2.
A 36-year-old female with thoracic myelopathy. The preoperative magnetic resonance imaging (MRI) (A) and computed tomography (B) show severe spinal cord compression and intramedullary signal changes at T8/9 with calcific disc herniation. Because of the adhesion between the dura, the calcified disc was floated without total removal (C) as seen on the MRI performed a few days postoperatively, but the patient had significant improvement after surgery and did not require additional surgery (D). SEP, superior end plate; LEP, lower end plate.
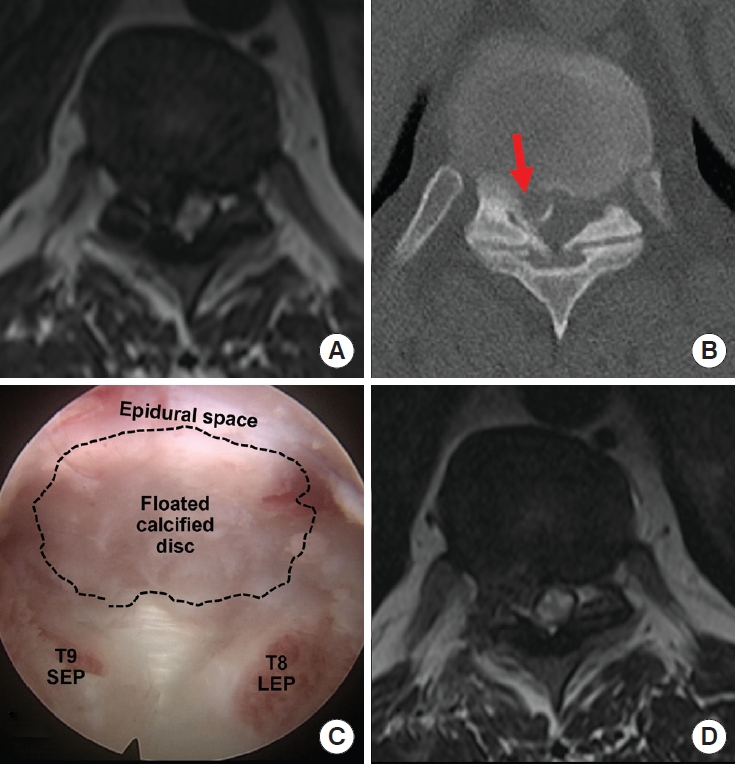
Fig. 3.
Axial magnetic resonance image demonstrates the location of portal (the entry of a discography needle) and access angle. The entry is located at around 5–8 cm from the midline, and the access angle is around 45° because of the rib cage.
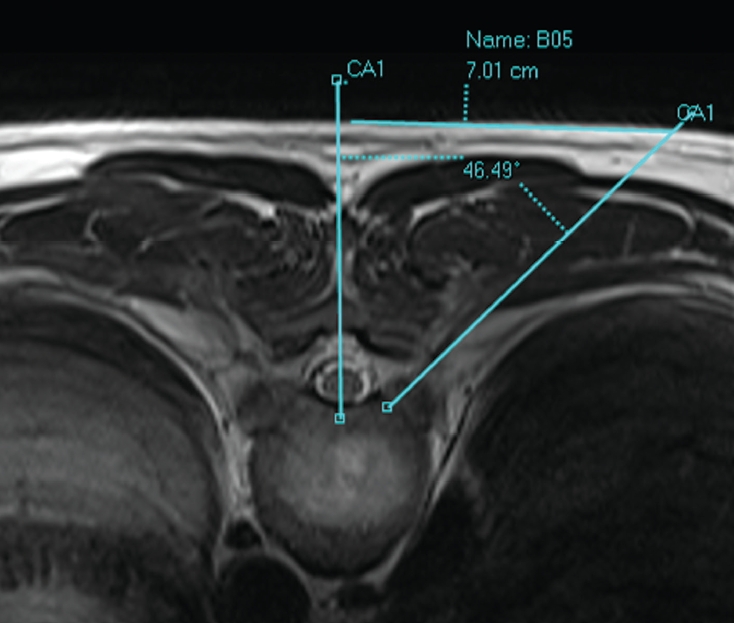
Fig. 4.
The initial discography needle and guide wide should touch the posterolateral corner of the intervertebral disc (A, B) on fluoroscopic images. (C, D) The obturator and working cannula is touching lateral aspect of the facet joint.
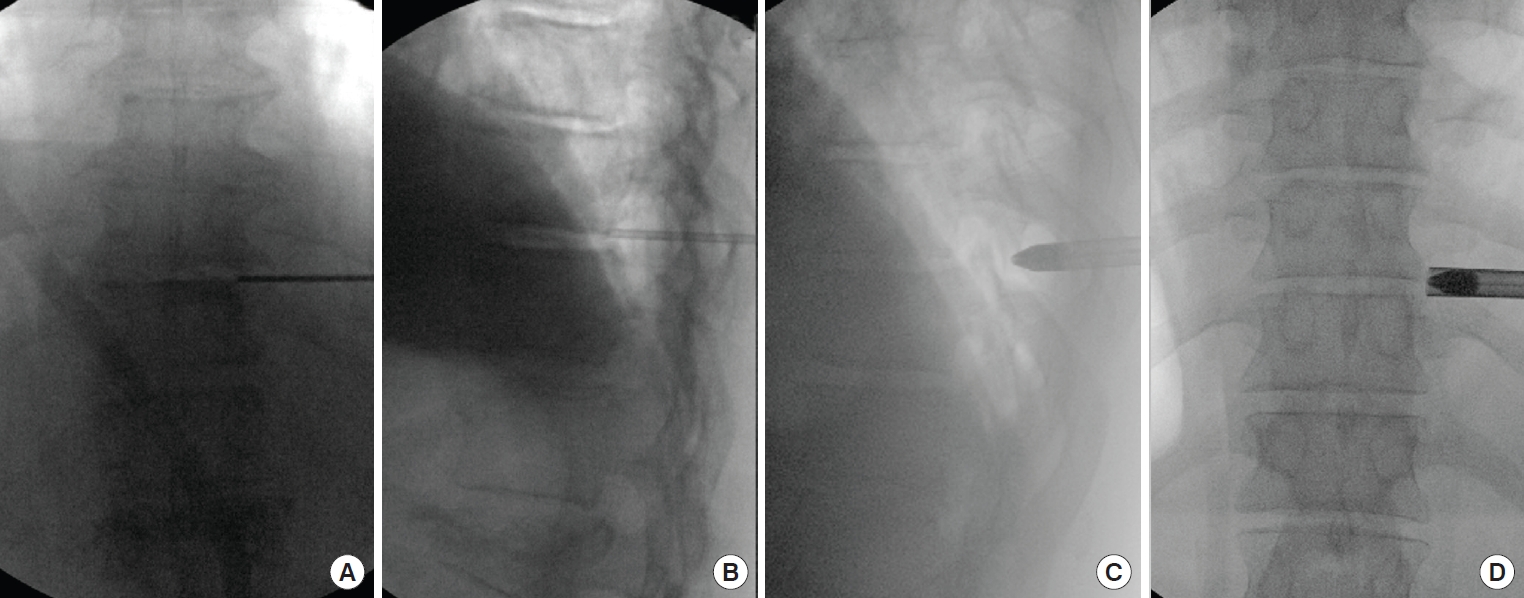
Fig. 5.
Computed tomography (CT) images demonstrate anatomical characteristics of the thoracic spine. (A) T10/11, T11/12 disc space is not covered by the corresponding rib heads (red arrows), but at the levels above T10, the disc space is partially covered by the rib heads (arrow head), drilling of the superior aspect of the rib head maybe needed to access the disc space, especially toward the central located disc fragment. Since pedicles of thoracic spine have a caudad angle, the upper part of the pedicle usually blocks access to the disc space (the dotted lines). Drilling of the upper pedicle-superior end plate junction provides easier access to the disc space. (B) Oblique view of a 3-dimensional CT images of the foramen. To expose the intervertebral foramen of thoracic spine, lateral facetectomy will be necessary, drilling of up to 50% of the joint surface, superior pediculectomy to the superior end plate, and superior aspect of the rib head as needed (red area). (C) An axial CT images shows lateral facetectomy and the superior end plate resection with an endoscopic burr (the red circle).

Fig. 6.
Intraoperative pictures of sequential steps showing exposure of a right side T9/10 intervertebral foramen and intervertebral disc space. (A) After soft tissue removal, lateral aspect of the inferior articular process (IAP) of the cranial vertebra was drilled. (B) After the IAP resection, the superior articular process (SAP) of the inferior vertebra was drilled to open the foramen. (C) Superior aspect of the lower pedicle was drilled to expose the disc space. (D) After lateral facetectomy and drilling of the superior pedicle, the upper (arrow heads) and lower end plate (arrows), the disc space was exposed.
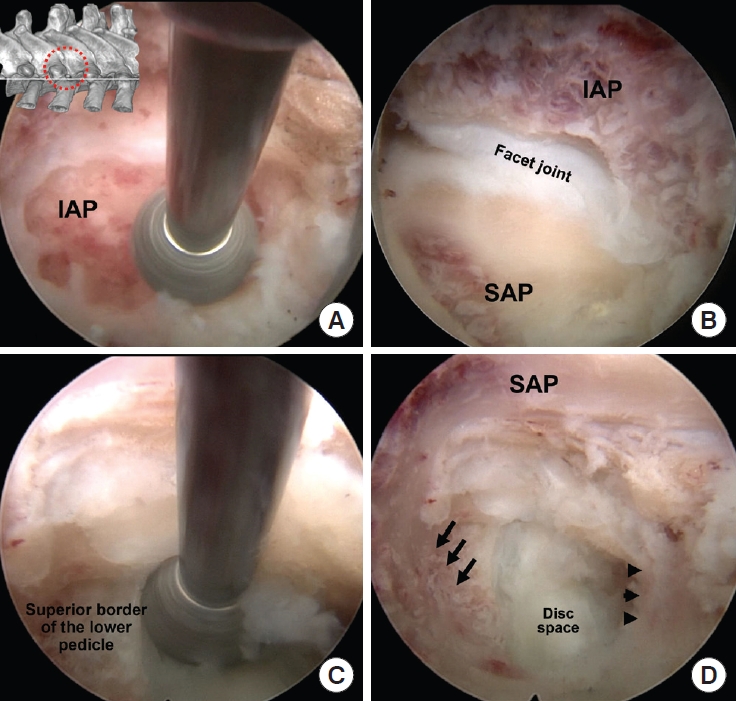
Fig. 7.
Intraoperative pictures of the decompression. (A) After posterolateral discectomy, the lateral border of dura was exposed. (B) A radiofrequency probe or a dissector can be placed between the ventral dura and posterior annulus to confirm the plane and check for any adhesion. (C, D) After central discectomy, the pulsating ventral dura and epidural fat are visible without any compression.
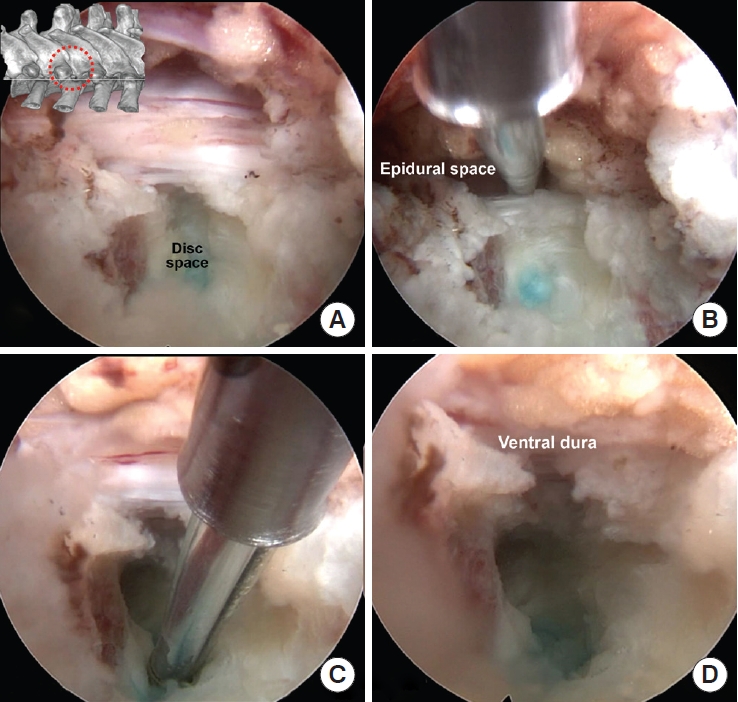
Table 1.
Summary of transforaminal endoscopic thoracic discectomy clinical series reviewed
| Study | Indication | No. of patients | Clinical outcomes* | Complications (n) |
|---|---|---|---|---|
| Choi et al., [4] 2010 | Soft thoracic disc herniation | 14 | VAS back 6.5 to 3 | Not reported |
| VAS leg 5.8 to 2.5 | ||||
| ODI 58.1 to 24.5 | ||||
| Follow-up 60.2 months | ||||
| Bae et al., [5] 2020 | Soft thoracic disc herniation | 92 | VAS 7.6 to 1.6 | Transient motor weakness (1), parasthesias (3), symptomatic recurrent herniations (2), reoperation (1) |
| ODI 68.2 to 13.2 | ||||
| MacNab excellent/good outcomes 90.2% | ||||
| Follow-up time 38.4 months | ||||
| Guo et al., [13] 2019 | Lower thoracic stenosis | 6 | JOA score 4.4 to 6.6 at 1 year | Not reported |
| VAS back 7.8 yo 1.9 | ||||
| VAS leg 8.7 to 0.3 | ||||
| Follow-up time 12.6 months | ||||
| Bae et al., [7] 2022 | Thoracic disc herniation | 39 | VAS 7.5 to 2.5 | Incomplete decompression requiring revision (1) |
| ODI 47.6 to 13.7 | ||||
| MacNab excellent/good outcomes 89.7% | ||||
| Follow-up time 11.2 months | ||||
| Bae et al., [16] 2019 | Upper thoracic disc herniation (T2–6) | 14 | VAS 7.3 to 2.3 | Not reported |
| ODI 53.5 to 16.9 | ||||
| MacNab excellent/good outcomes (86%) | ||||
| Follow-up 43.4 months | ||||
| Houra et al., [18] 2020 | Thoracic disc herniations (including 10 calcified) | 16 | VAS 8 to 1 | Not reported |
| ODI 59 to 13 | ||||
| Follow-up 5 years | ||||
| Gao et al., [6] 2021 | Thoracic disc herniations (including 9 calcified) | 11 | JOA from 7.4 to 10.2 | Not reported |
| VAS leg/thoracic 3 to 0.5 | ||||
| Follow-up time 15 months |
REFERENCES
1. Arseni C, Nash F. Thoracic intervertebral disc protrusion: a clinical study. J Neurosurg 1960;17:418-30.

2. Uribe JS, Smith WD, Pimenta L, et al. Minimally invasive lateral approach for symptomatic thoracic disc herniation: initial multicenter clinical experience. J Neurosurg Spine 2012;16:264-79.


3. Quint U, Bordon G, Preissl I, et al. Thoracoscopic treatment for single level symptomatic thoracic disc herniation: a prospective followed cohort study in a group of 167 consecutive cases. Eur Spine J 2012;21:637-45.



4. Choi KY, Eun SS, Lee SH, et al. Percutaneous endoscopic thoracic discectomy; transforaminal approach. Minim Invasive Neurosurg 2010;53:25-8.


5. Bae J, Chachan S, Shin SH, et al. Transforaminal endoscopic thoracic discectomy with foraminoplasty for the treatment of thoracic disc herniation. J Spine Surg 2020;6:397-404.



6. Gao S, Wei J, Li W, et al. Full-endoscopic transforaminal ventral decompression for symptomatic thoracic disc herniation with or without calcification: technical notes and case series. Pain Res Manag 2021;2021:6454760.




7. Bae J, Kim J, Lee SH, et al. Comparative analysis of transforaminal endoscopic thoracic discectomy and microscopic discectomy for symptomatic thoracic disc herniation. Neurospine 2022;19:555-62.




8. Wagner R, Telfeian AE, Iprenburg M, et al. Transforaminal endoscopic foraminoplasty and discectomy for the treatment of a thoracic disc herniation. World Neurosurg 2016;90:194-8.


9. Rosenthal D. Endoscopic approaches to the thoracic spine. Eur Spine J 2000;9 Suppl 1(Suppl 1):S8-16.



10. Kambin P, Gellman H. Percutaneous lateral discectomy of the lumbar spine a preliminary report. Clin Orthop Relat Res (1976-2007) 1983;174:127-32.
11. Khandge AV, Sharma SB, Kim JS. The evolution of transforaminal endoscopic spine surgery. World Neurosurg 2021;145:643-56.


12. Moraes Amato MC, Aprile BC, Esteves LA, et al. Full endoscopic thoracic discectomy: is the interlaminar approach an alternative to the transforaminal approach? A technical note. Int J Spine Surg 2022;16:309-17.



13. Guo C, Zhu D, Kong Q, et al. Transforaminal percutaneous endoscopic decompression for lower thoracic spinal stenosis. World Neurosurg 2019;128:e504-12.


14. Azad TD, Alomari S, Khalifeh JM, et al. Adoption of awake spine surgery - trends from a national registry over 14 years. Spine J 2022;22:1601-9.


15. Hofstetter CP, Ahn Y, Choi G, et al. AOSpine Consensus Paper on nomenclature for working-channel endoscopic spinal procedures. Global Spine J 2020;10(2 Suppl):111S-121S.




16. Bae J, Chachan S, Shin SH, et al. Percutaneous Endoscopic thoracic discectomy in the upper and midthoracic spine: a technical note. Neurospine 2019;16:148-53.




17. Choi G, Munoz-Suarez D. Transforaminal Endoscopic thoracic discectomy: technical review to prevent complications. Neurospine 2020;17(Suppl 1):S58-65.




18. Houra K, Saftic R, Knight M. Five-year outcomes after transforaminal endoscopic foraminotomy and discectomy for soft and calcified thoracic disc herniations. Int J Spine Surg 2021;15:494-503.



19. Houra K, Saftic R. Transforaminal endoscopic discectomy for large, two level calcified, thoracic disc herniations with 5-year follow-up. Neurospine 2020;17:954-9.




20. Ruetten S, Hahn P, Oezdemir S, et al. Operation of soft or calcified thoracic disc herniations in the full-endoscopic uniportal extraforaminal technique. Pain Physician 2018;21:E331-40.


21. Zhang LM, Lv WY, Cheng G, et al. Percutaneous endoscopic decompression for calcified thoracic disc herniation using a novel T rigid bendable burr. Br J Neurosurg 2019 Jan;28:1-3. doi:10.1080/02688697.2018.1557593. [Epub].

22. Sclafani JA, Kim CW. Complications associated with the initial learning curve of minimally invasive spine surgery: a systematic review. Clin Orthop Relat Res 2014;472:1711-7.



23. Hsu HT, Chang SJ, Yang SS, et al. Learning curve of full-endoscopic lumbar discectomy. Eur Spine J 2013;22:727-33.




- TOOLS






























