- Search
|
|
||
Abstract
Objective
Expandable cage technology has emerged for lumbar interbody fusion to restore intervertebral disc space height and alignment through a narrow surgical corridor. The purpose of this study is to present the technique of biportal endoscopic transforaminal lumbar interbody fusion (TLIF) using dual direction expandable cage and provide early clinical results.
Methods
We performed the biportal endoscopic TLIF using a dual direction expandable titanium cage for height restoration and a larger footprint in 10 patients. Clinical parameters including Oswestry Disability Index (ODI), visual analogue scale (VAS), and complications were retrospectively analyzed. Also, we investigated radiologic parameters using preoperative and postoperative x-ray images.
Results
We successfully inserted dual direction expandable cages during biportal endoscopic TLIF. There was no significant subsidence or collapse of the expandable cages during the 6-month follow-up period. Lumbar lordosis and disc height were significantly increased after surgery. ODI and VAS scores were significantly improved at 6 months after surgery.
Minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) has demonstrated comparable clinical outcomes and safety profile as compared to open conventional TLIF with significant improvement of pain and disability [1,2]. More recently, endoscopic techniques to perform TLIF surgery have been introduced with similar success as MIS-TLIF, especially with biportal endoscopic techniques [3-8]. The biportal endoscopic TLIF technique is similar to the MIS-TLIF technique in that the technique utilizes a posterolateral interlaminar approach, while visualizing the spinal anatomy with an endoscopic camera [7-9]. Through the technique, direct decompression of the spinal canal can be achieved and interbody fusion can be completed through a transforaminal approach. This allows for restoration of intervertebral disc height and reduction of the spondylolisthesis, which has demonstrated significant correlation with clinical success [10,11]. The biportal endoscopic technique is less invasive as compared to other MIS techniques with preservation of the lumbar musculoligamentous structures, which may reduce postoperative pain and facilitate recovery [5,7,8,12].
Expandable cage technology has been developed for interbody fusion and has demonstrated the ability to restore intervertebral disc height and correct alignment [13,14]. However, subsidence of the vertebral endplates is a significant concern, especially with point loading of a narrow cage within the center of the intervertebral disc space [15,16]. A narrow cage is typically utilized for a TLIF approach due to the narrow corridor available within the neural foramen to introduce the implant. With subsidence, collapse of disc height, loss of reduction, and malalignment may occur, which can lead to suboptimal clinical outcomes. Recently, a novel dual direction expandable titanium TLIF cage has been developed that expands both in the medial to lateral dimension and in height. The cage can be placed through the neural foramen in the narrow, collapsed state. Once in the disc space, the medial to lateral expansion increases the surface area of endplate bony contact and provides contact with the apophyseal rings, which has been shown to be the strongest portion of the vertebral endplate [17,18]. With these advantages, complete expansion with this dual expandable cage may lead to less subsidence and restore lumbar lordosis.
The purpose of this study is to present the technique of biportal endoscopic TLIF utilizing the dual direction expandable titanium TLIF cage and provide preliminary results.
We enrolled patients who were obtained single level biportal endoscopic TLIF using the dual direction expandable TLIF cage (Dual-X TLIF, Amplify Surgical, Inc., Irvine, CA, USA) in this study (Fig. 1). The design of this study was a retrospective analysis of prospectively collected data with description of surgical technique. After obtaining Institutional Review Board (IRB) approval from the hospital where the author was affiliated (IRB approval No. CA-TR-1), the investigations was performed. The design of this study was a technical report with preliminary data. The indications of this TLIF technique included degenerative spondylolisthesis, lumbar central stenosis, Lumbar foraminal stenosis and isthmic spondylolisthesis. We excluded the revision surgery, infection, trauma, and multilevel disease. Only patients who had full clinical and radiographic data for at least 6 months after surgery were included in the study.
We analyzed clinical data including Oswestry Disability Index (ODI), visual analogue scale (VAS) of back and leg, operation time, estimated blood loss, and complications. Estimated blood loss included postoperative blood drainage amount. We obtained lumbar radiographs, including anteriorposterior (AP) and lateral x-rays including flexion and extension lateral views preoperatively, immediately postoperatively and 6 months after surgery. We measured disc height of operative segment (anterior height+posterior height/2), segmental lordotic angle of operative level, and lumbar lordotic angle using preoperative and postoperative x-rays. Significant cage subsidence was defined as a cage invading the vertebral body by more than 2 mm. Subsidence and collapse of the expandable cages were evaluated by disc height measurement.
Since the patient sample was small, nonparametric statistics were used. Statistical analysis was performed using Wilcoxon signed-rank test, and Kruskal-Wallis test. A p < 0.05 was considered to be statistically significant. R 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
The procedure utilizes biportal endoscopy, which consists of an endoscopic camera, endoscopic irrigation equipment, monitor, radiofrequency (RF) console with probes, high speed bur, bone cutting endoscopic shaver device, and standard surgical instruments [4,9,12].
The dual direction expandable titanium TLIF cages start at a height of 7 mm that expand to 3-mm increments and width of 12 mm that expand to 21 mm with cage length options of 25 and 30 mm (Fig. 1). The cages are available in 0┬░, 8┬░, 12┬░, and 15┬░ lordotic options. The cage is designed with a large center chamber for bone graft placement after expansion and an open structure design that allows bone graft to be placed through the cage and into the disc space. The cage is designed with 2 independent locking mechanisms to ensure that the cage remains expanded in both width and height. Initial locking occurs with an expansion locking mechanism and a secondary active locking occurs with insertion of a locking screw through the cage.
We preferred general endotracheal anesthesia for biportal endoscopic TLIF. After anesthesia, the patient is placed in the prone position on a Jackson table or a Wilson frame. Two incisions are made for the biportal endoscopic procedure (Fig. 2A). The first incision is made over the ipsilateral caudal pedicle below the disc space as the working portal, measuring approximately 2 cm (Fig. 2B). The surgical instruments, outflow cannula, interbody cage, and pedicle screw can all be introduced through this working portal. The second incision is for the viewing portal, which is a 5-mm stab incision made approximately 2 cm cephalad to the working portal and lateral to the pedicle (Fig. 2B). Two 18-gauge 90-mm length spinal needles are initially placed through the planned incision sites. Lateral fluoroscopic images are used to verify the correct spinal level and disc space as well as trajectories. Once the working portal incision is made, the lumbodorsal fascia is incised in the trajectory of the portal and serial dilators are inserted. The paraspinal musculature and adventitia are bluntly dissected off the cephalad and caudal laminae and a working space is created over the laminae. An outflow cannula is then placed in the working portal and the endoscopic camera is introduced after creating the viewing portal. After the endoscopic irrigation is started, the endoscopic camera and a RF probe are then triangulated over the cephalad lamina (Fig. 3A). Basically, our biportal endoscopic TLIF is similar to MISTLIF using tubular retractor systems. At this point, if patients have symptomatic central stenosis, a unilateral laminotomy with bilateral decompression can be performed as previously described (Fig. 3B) [4,6]. After the decompression is complete, a complete facetectomy is performed with a straight osteotome under direct visualization of the endoscope. The bone from the facetectomy can be harvested and processed as autograft for later in the procedure. Once the disc space is identified, and the annulus fibrosis is then incised by a blunt annular knife. Serial disc space shavers are then introduced into the disc space to remove the disc material and cartilaginous endplate. The disc material can then be further removed with a series of pituitaries and angled curettes under direct endoscopic visualization (Fig. 4A). The complete preparation of the bony endplates with bleeding bony surfaces can be verified directly by the endoscope (Fig. 4B). Prior to placing the final implant, serial trials are inserted into the disc space to determine the initial and final height that the disc space can accommodate. Only after proper trialing, the final implant is then selected.
Autograft can be introduced into the disc space using a specialized endoscopic funnel. The collapsed dual direction expandable cage is then inserted into the disc space with retraction of the thecal sac, traversing and exiting nerve root using specialized endoscopic retractors (Figs. 5, 6). A customized cage guidance helps to safely insert the cage into disc space. The cage is impacted to the anterior border of the disc space and across the midline under fluoroscopic guidance in both the AP and lateral projections (Fig. 5A, B). The cage is expanded initially in the medial to lateral direction (Fig. 5C). Once this is complete, the cage is then expanded to the final height position (Fig. 5D). After inserting the cage into the disc space, turning the insertion handle will initially expand the cage in the medial to lateral direction to the final width of 21 mm for increased surface area covered within the disc space. Once medial to lateral expansion is complete, then cage height expansion proceeds. The final height was previously determined by the trialing and the cage will expand in height by 3 mm to the final height with continued rotation of the insertion handle. Proper trialing and cage selection is paramount to prevent endplate damage and subsidence.
The secondary locking screw is then inserted and locked into final position. The inserter is then removed from the cage and fluoroscopic images are obtained in the AP and lateral projections.
Specialized bone graft cannulas are filled with allograft material such as demineralized bone matrix (DBM) putty and fiber and the cannulas are used to introduce the allograft material into the cage and disc space. The open architecture of the cage allows for the allograft to freely fill the cage and disc space. Typically, endoscopic fluid irrigation is paused during the insertion of the allograft material. A surgical drain is then placed into the laminotomy site to reduce the risk of epidural hematoma postoperatively. All endoscopic equipment is then removed, and percutaneous pedicle screws are placed in the standard fashion like MIS-TLIF (Fig. 6).
We successfully performed biportal endoscopic TLIF surgeries using dual direction expandable cages in 10 patients. All surgeries included biportal endoscopic unilateral laminotomy, bilateral decompression with TLIF and percutaneous pedicle screw fixation as described. The average age was 68.5 ┬▒ 5.4 years old with 6 females and 4 males. The diagnoses included degenerative spondylolisthesis with concomitant central stenosis (9 cases) and isthmic spondylolisthesis (1 case). The levels involved included L4ŌĆō5 (8 cases), L5ŌĆōS1 (2 cases). The average operation time was 151.4 ┬▒ 30.6 minutes. The mean postoperative estimated blood loss as measured by drain output was 156.6 ┬▒ 74.2 mL (Table 1).
Preoperative VAS of back decreased significantly from 6.9 ┬▒ 1.19 to 2.1 ┬▒ 1.85 at 6 weeks postoperatively, 1.3 ┬▒ 1.57 at 3 months postoperatively, and 1.25 ┬▒ 0.63 at 6 months after surgery (p < 0.05). Preoperative VAS of leg decreased significantly from 8.3 ┬▒ 1.16 to 0.55 ┬▒ 1.57 at 6 weeks postoperatively, 1.6 ┬▒ 1.65 at 3 months postoperatively, and 1 ┬▒ 0.94 at 6 months after surgery (p < 0.05). Preoperative ODI significantly improved from 55.2 ┬▒ 9.1 to 32.3 ┬▒ 17.3 at 6 weeks postoperatively, 29.1 ┬▒ 15.5 at 3 months postoperatively, and 26.6 ┬▒ 7.5 at 6 months after surgery (p < 0.05) (Table 2). There was one complication with an epidural hematoma causing a right ankle dorsiflexion weakness (G 3 of 5) postoperatively that required evacuation of the epidural hematoma on postoperative one day. After epidural hematoma removal, ankle weakness recovered well. Otherwise, there were no incidental durotomies, wound infections, implant failures, or medical complications in this clinical series.
Intervertebral disc height of operation segment was significantly widened and well maintained. The mean disc height of operation segment was significantly increased from 5.7 ┬▒ 2.7 mm to 13.2 ┬▒ 1.1 mm immediately after surgery, and 12.6 ┬▒ 1.1 mm at 6 months after surgery (p < 0.05). Also, preoperative segmental lordotic angle and lumbar lordotic angle were significantly increased and well maintained at 6 months after surgery (p < 0.0.5) (Table 3).
Postoperative radiographs at 6-month follow-up demonstrated no malposition or instrument failure with the cages or pedicle screws. There were no significant subsidence or recollapse of inserted cages.
With advancements in cage technology, many types of expandable cages have been developed for lumbar interbody fusion surgery. However, one of the main issues and criticisms of expandable TLIF cages is the point loading of the endplate due to the narrow cage geometry and differing modulus of elasticity of titanium to bone that may contribute to subsidence [15,16,19]. This is especially true with osteopenic and osteoporotic bone, which is commonly seen in the older patient population that typically suffer from lumbar spondylolisthesis and stenosis.
The dual direction expandable titanium TLIF cage is a novel implant design that creates a wider footprint after placement within the disc space. Since the cage is initially in the collapsed and smaller state, it can be introduced endoscopically without difficulty. The wider footprint after initial expansion allows for greater surface area of vertebral endplate contact, which is advantageous for both disc height restoration and fusion purposes. The geometry of the cage contacts the anterior and posterior apophyseal ring, which is the stronger regions of the vertebral endplates, potentially reducing the risk of subsidence. With its open architecture, bone graft material such as flowable DBM allograft fibers can easily be packed into the cage and disc space after insertion of the cage. Alignment correction is achievable with the various lordosis options available for the cage. When performing endoscopic TLIF, it can be difficult for the surgeon to insert a standard cage using a small skin incision. In addition, neural injury may occur when a large-sized cage is inserted through the neural foramen during endoscopic TLIF. However, using an expandable cage may make it easier and safer to insert the cage in endoscopic TLIF. When inserting a large interbody cage in MIS-TLIF or endoscopic TLIF, nerve root injury is a concern given the anatomical constraints. On the other hand, inserting a cage that is too small can result in fusion failure or cage pullout. The dual expandable cage is inserted in a small state and expanded to a large state in 2 dimensions within the disc space, which can prevent pullout and subsidence from occurring. Therefore, if a dual expandable cage is used in biportal endoscopic TLIF, the cage can be safely inserted without damaging the nerve root, and complications associated with cage implant failure can be minimized. Although the expandable cages have various advantages compared to the static cages, longterm research is needed. A comparative study using a large cohort and long-term follow-up is needed to elucidate the advantages of an expandable cages compared to a static cage.
Biportal endoscopic TLIF combines the advantages of endoscopic spine surgery and the enhanced visualization using the endoscope with the advantages of MIS-TLIF. Although the experience is still early with biportal endoscopic TLIF, several studies have demonstrated the clinical effectiveness and safety of the technique, demonstrating the technique is similar in the clinical outcomes as compared to MIS-TLIF at 1-year follow-up [3,5,7,8]. Our early clinical experience of the initial 10 patients with at least 6-month follow-up demonstrated improvement of both back and leg pain as well as disability as compared to the preoperative state with no complications seen on postoperative radiographs. We did experience one case of epidural hematoma that necessitated reoperation with evacuation of the hematoma. Epidural hematoma is a known complication of biportal endoscopic TLIF due to more extensive bone work that leads to bony bleeding into a small, contained space within the spinal canal [8]. Given this, the routine use of postoperative drains is advocated to reduce the risk of epidural hematoma in these cases [20].
The advantage of the biportal endoscopic TLIF is the minimally invasive nature of the surgery with very small incisions, minimal soft tissue trauma, yet without compromise of clinical effectiveness. The posterolateral interlaminar approach used in biportal endoscopic TLIF is very familiar to spine surgeons, whether they are trained in open or MIS surgery [4,8]. Complete and thorough spinal canal decompression can be performed even with severe stenosis that is often seen concurrently with spondylolisthesis in these patients. In addition, there is less risk of damage to the exiting and traversing nerve roots with the transforaminal approach as long as sufficient space is created with the laminotomy, decompression, and facetectomy [8,9]. Another key advantage is the direct visualization and confirmation of a full endplate preparation using the endoscope and instruments such as angled curettes and pituitaries used within the disc space along with the endoscope. Proper and complete endplate preparation is a crucial step in achieving successful arthrodesis with the TLIF technique, whether it be open, MIS, or endoscopic [6,7]. Prior studies have demonstrated that traditional TLIF techniques remove suboptimal disc material during the discectomy and the endplates may be insufficiently prepared during the procedure [21,22]. This may lead to lower fusion rates and worse clinical outcomes over the long-term since successful arthrodesis has been correlated with clinical success [23]. The verification of complete discectomy and endplate preparation with the endoscope may contribute to higher fusion rates based on the extent and completeness of the preparation. Multiple studies have shown that successful clinical outcomes after lumbar fusion are correlated with successful arthrodesis, disc height restoration, and alignment correction [24-26].
There were several limitations of this study. Since this study focused as a novel technical note of biportal endoscopic TLIF using the dual direction titanium expandable cage, the number of patients was small and follow-up period was short. This study is not a comparative study, but a preliminary study that described a small case series. Therefore, in order to fully investigate the clinical effects of expandable cages in biportal endoscopic TLIF, larger, long-term multi-center prospective studies and randomized case control studies are necessary.
In this study, we introduced the novel technique of inserting a dual direction expandable cage with biportal endoscopic TLIF. This is the first description of its kind in the scientific literature. We successfully performed the insertion of a dual direction expandable cage in biportal endoscopic TLIF. In the preliminary results, the radiographic and clinical outcomes may be favorable. All inserted expanded cages were well maintained without significant collapse or subsidence in our early experience. Biportal endoscopic TLIF using a dual direction expandable cage may be a successful alternative surgical option for treatment of lumbar degenerative disease.
NOTES
Fig.┬Ā1.
Pictures of the dual expandable titanium cage in the fully collapsed state and the fully expanded state. Fully collapsed (A), width expansion (B), and height expansion (C).

Fig.┬Ā2.
(A) Overview of biportal endoscopic approach. Intraoperative photograph depicting the endoscope placed in the viewing portal and the surgical instrument placed in the working portal. (B) Intraoperative anteriorposterior fluoroscopy image depicting the location of the portals. The white line is the location of the viewing endoscopic portal and the black line is the location of the working portal.
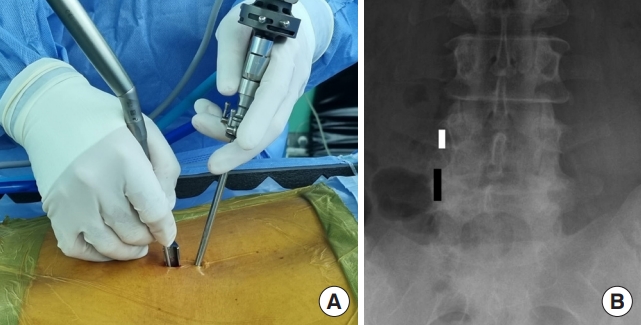
Fig.┬Ā3.
(A) Intraoperative fluoroscopy image showing the endoscopic camera and radiofrequency probe triangulated over the L4 lamina and disc space of L4ŌĆō5. (B) Intraoperative endoscopic photograph showing the dura and traversing nerve root exposed after completion of the unilateral laminotomy and bilateral decompression.
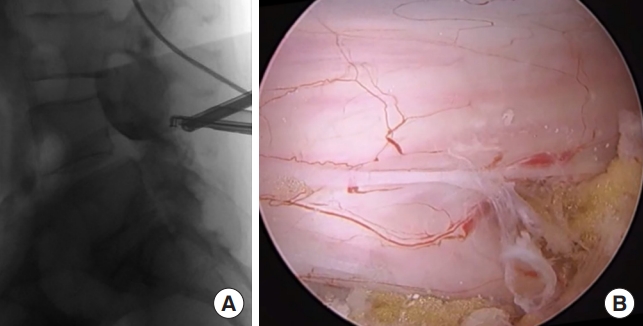
Fig.┬Ā4.
(A) Intraoperative lateral fluoroscopy image showing the endoscopic camera within the intervertebral disc space during the discectomy and endplate preparation with an angled curette. (B) Intraoperative endoscopic photograph showing the intervertebral disc space after complete discectomy and endplate preparation with removal of the cartilaginous endplate for fusion.

Fig.┬Ā5.
Intraoperative anteroposterior (A) and lateral (B) fluoroscopy image during the initial placement of the dual expandable titanium cage into the disc space with endoscopic visualization. The cage has been placed near the ventral aspect of the disc space. (C) Intraoperative anteriorposterior fluoroscopy image after the cage has been fully expanded in the medial to lateral dimension in the midline of the disc space. (D) Lateral fluoroscopy image after the cage has been fully expanded in height.
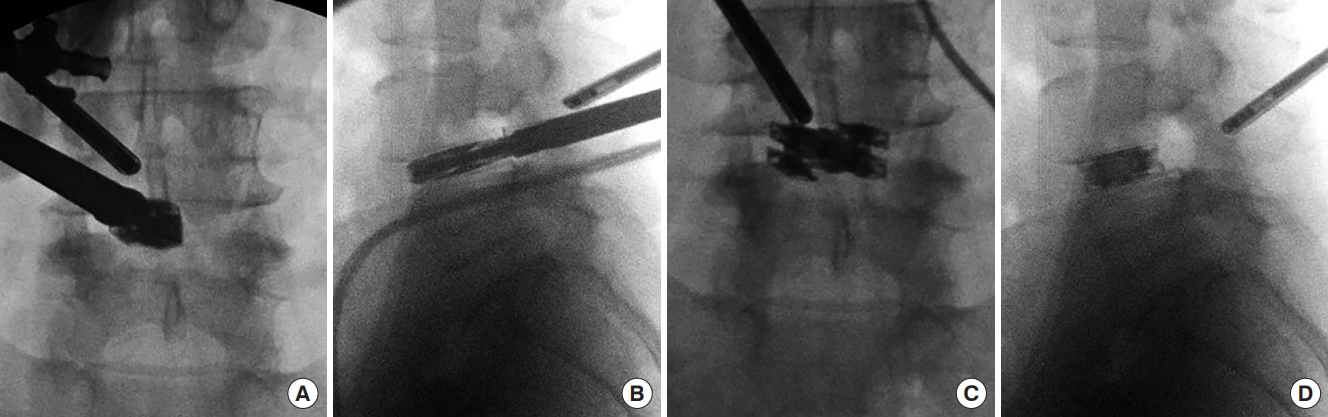
Fig.┬Ā6.
A 63-year-old female presented with low back pain, left lower extremity. Biportal endoscopic transforaminal lumbar interbody fusion with unilateral laminotomy with bilateral decompression using a dual direction expandable titanium cage was performed with a left sided approach. Preoperative anteriorposterior (AP) (A) and lateral (B) x-ray images showing lower lumbar degenerative changes, facet arthropathy and grade 1 L4ŌĆō5 spondylolisthesis with disc space narrowing. (C) Preoperative axial magnetic resonance imaging image demonstrating L4ŌĆō5 severe central stenosis, facet and ligamentum hypertrophy. Intraoperative AP (D) and lateral (E) fluoroscopy images showed that dual expandable cage is inserted at L4ŌĆō5 disc space. Intervertebral space is expanded after a cage insertion. Pedicle screws were placed with bone cement augmentation. Postoperative AP (F) and lateral (G) x-ray images taken 6 months after surgery revealed that the cage expansion was well maintained without subsidence or recollapse.

Table┬Ā1.
Characteristics of patients
Table┬Ā2.
Clinical results
| Variable | Preoperative |
Postoperative |
||
|---|---|---|---|---|
| 6 Weeks | 3 Months | 6 Months | ||
| VAS back* | 6.9 ┬▒ 1.19 | 2.1 ┬▒ 1.85 | 1.3 ┬▒ 1.57 | 1.25 ┬▒ 0.63 |
| VAS leg* | 8.3 ┬▒ 1.16 | 0.55 ┬▒ 1.57 | 1.6 ┬▒ 1.65 | 1.0 ┬▒ 0.94 |
| ODI* | 55.2 ┬▒ 9.1 | 32.3 ┬▒ 17.3 | 29.1 ┬▒ 15.5 | 26.6 ┬▒ 7.5 |
Table┬Ā3.
Radiographic results
| Variable | Preoperative |
Postoperative |
|
|---|---|---|---|
| Immediate | 6 Months | ||
| Disc height of operative segment (mm)* | 5.7 ┬▒ 2.7 | 13.2 ┬▒ 1.1 | 12.6 ┬▒ 1.1 |
| Lordotic angle of operative segment (┬░)* | 17.6 ┬▒ 7.7 | 21.1 ┬▒ 6.2 | 20.3 ┬▒ 6.0 |
| Lumbar lordotic angle (┬░)* | 34.3 ┬▒ 6.2 | 41.1 ┬▒ 2.6 | 42.9 ┬▒ 4.7 |
REFERENCES
1. Heemskerk JL, Oluwadara Akinduro O, Clifton W, et al. Long-term clinical outcome of minimally invasive versus open single-level transforaminal lumbar interbody fusion for degenerative lumbar diseases: a meta-analysis. Spine J 2021;21:2049-65.


2. Jin-Tao Q, Yu T, Mei W, et al. Comparison of MIS vs. open PLIF/TLIF with regard to clinical improvement, fusion rate, and incidence of major complication: a meta-analysis. Eur Spine J 2015;24:1058-65.



3. Kang MS, Heo DH, Kim HB, et al. Biportal endoscopic technique for transforaminal lumbar interbody fusion: review of current research. Int J Spine Surg 2021;15(suppl 3):S84-92.



4. Heo DH, Hong YH, Lee DC, et al. Technique of biportal endoscopic transforaminal lumbar interbody fusion. Neurospine 2020;17(Suppl 1):S129-37.




5. Kang MS, You KH, Choi JY, et al. Minimally invasive transforaminal lumbar interbody fusion using the biportal endoscopic techniques versus microscopic tubular technique. Spine J 2021;21:2066-77.


6. Heo DH, Son SK, Eum JH, et al. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurg Focus 2017;43:E8.


7. Heo DH, Park CK. Clinical results of percutaneous biportal endoscopic lumbar interbody fusion with application of enhanced recovery after surgery. Neurosurg Focus 2019;46:E18.


8. Heo DH, Lee DC, Kim HS, et al. Clinical results and complications of endoscopic lumbar interbody fusion for lumbar degenerative disease: a meta-analysis. World Neurosurg 2021;145:396-404.


9. Heo DH, Eum JH, Jo JY, et al. Modified far lateral endoscopic transforaminal lumbar interbody fusion using a biportal endoscopic approach: technical report and preliminary results. Acta Neurochir (Wien) 2021;163:1205-9.



10. Ha KY, Na KH, Shin JH, et al. Comparison of posterolateral fusion with and without additional posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Spinal Disord Tech 2008;21:229-34.


11. McAfee PC, DeVine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976) 2005;30(6 Suppl):S60-5.
12. Heo DH, Lee DC, Park CK. Comparative analysis of three types of minimally invasive decompressive surgery for lumbar central stenosis: biportal endoscopy, uniportal endoscopy, and microsurgery. Neurosurg Focus 2019;46:E9.

13. Hawasli AH, Khalifeh JM, Chatrath A, et al. Minimally invasive transforaminal lumbar interbody fusion with expandable versus static interbody devices: radiographic assessment of sagittal segmental and pelvic parameters. Neurosurg Focus 2017;43:E10.

14. Alvi MA, Kurian SJ, Wahood W, et al. Assessing the difference in clinical and radiologic outcomes between expandable cage and nonexpandable cage among patients undergoing minimally invasive transforaminal interbody fusion: a systematic review and meta-analysis. World Neurosurg 2019;127:596-606.e1.


15. Chang CC, Chou D, Pennicooke B, et al. Long-term radiographic outcomes of expandable versus static cages in transforaminal lumbar interbody fusion. J Neurosurg Spine 2020 Nov 13:1-10. doi: 10.3171/2020.6.SPINE191378. [Epub].


16. Armocida D, Pesce A, Cimatti M, et al. Minimally invasive transforaminal lumbar interbody fusion using expandable cages: increased risk of late postoperative subsidence without a real improvement of perioperative outcomes: a clinical monocentric study. World Neurosurg 2021;156:e57-63.


17. Lowe TG, Hashim S, Wilson LA, et al. A biomechanical study of regional endplate strength and cage morphology as it relates to structural interbody support. Spine (Phila Pa 1976) 2004;29:2389-94.


18. Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine (Phila Pa 1976) 2000;25:1077-84.


19. Niinomi M, Liu Y, Nakai M, et al. Biomedical titanium alloys with Young's moduli close to that of cortical bone. Regen Biomater 2016;3:173-85.



20. Heo DH, Park DY, Hong HJ, et al. Indications, contraindications, and complications of biportal endoscopic decompressive surgery for the treatment of lumbar stenosis: a systematic review. World Neurosurg 2022;168:411-20.


21. Tatsumi R, Lee YP, Khajavi K, et al. In vitro comparison of endplate preparation between four mini-open interbody fusion approaches. Eur Spine J 2015;24 Suppl 3:372-7.



22. Rihn JA, Gandhi SD, Sheehan P, et al. Disc space preparation in transforaminal lumbar interbody fusion: a comparison of minimally invasive and open approaches. Clin Orthop Relat Res 2014;472:1800-5.



23. Djurasovic M, Glassman SD, Dimar JR 2nd, et al. Does fusion status correlate with patient outcomes in lumbar spinal fusion? Spine (Phila Pa 1976) 2011;36:404-9.


24. Levin JM, Tanenbaum JE, Steinmetz MP, et al. Posterolateral fusion (PLF) versus transforaminal lumbar interbody fusion (TLIF) for spondylolisthesis: a systematic review and meta-analysis. Spine J 2018;18:1088-98.

































