- Search
| Neurospine > Volume 20(3); 2023 > Article |
|
|
Abstract
Objective
This study aimed to analyze the clinical characteristics, treatment strategies, and surgical outcomes of subependymoma patients from the 2022 Neurospinal Society of Japan multicenter intramedullary spinal cord tumor study.
Methods
Twenty-six patients with spinal cord subependymoma who were included in the index study of 1,033 patients were retrospectively analyzed.
Results
Mean patient age was 49.4 years. Seventeen patients were men and 9 were women. Sensory disturbance was reported in 22 patients and motor weakness in 18. Median duration of symptoms was 24 months. The tumor was eccentrically located in 19 patients (73.1%) and unilateral in 17 (65.4%). Gross total resection was achieved in 6 patients (23.1%). The same rate for ependymoma patients in the index study was significantly higher (74.8%). Median follow-up was 40.5 months (interquartile range, 18–68 months). In 2 patients who underwent only partial resection, reoperation was required owing to progression 68 and 90 months after surgery, respectively. No recurrence occurred in patients who underwent gross total resection. Five patients experienced neurological worsening after surgery.
Conclusion
Although spinal cord subependymoma can be difficult to distinguish from other intramedullary spinal cord lesions before surgery, it is characterized by an indolent clinical course and eccentric location. Surgical treatment should prioritize functional preservation because the prognosis is good even after subtotal resection.
Subependymomas are rare, slow-growing, benign central nervous system tumors which predominantly occur in the fourth and lateral ventricles of the brain. Approximately 11% occur in the spinal cord [1-4]. Only 113 cases of spinal cord subependymoma have been reported since 1954 [1,5-32]; therefore, data regarding appropriate treatment, surgical outcomes, and long-term prognosis are limited. Although this tumor is a histologically benign lesion and can be cured by complete surgical resection, a surgical strategy that considers the preservation of neurological function is required at the same time. To date, both reports recommending complete resection [8] and safe maximal resection preserving neurological function [6,33] have been reported, but standard surgical strategies have not been established due to the limited number of patients. In 2022, the Neurospinal Society of Japan (NSJ) published the results of a retrospective multicenter cohort study of patients with intramedullary spinal cord tumors who underwent surgical treatment [34]. We aimed to analyze the clinical characteristics, treatment strategies, and surgical outcomes of the patients from this study who harbored a spinal cord subependymoma.
The NSJ study was approved by the institutional review board of the Tohoku University Hospital (2021-1-130) and 57 participating centers (listed in Acknowledgments section). The requirement for written informed consent was waived because the study was retrospective in design. Public notification of the study was provided on the websites of the individual centers. Data were recorded from 1,033 consecutive patients who underwent surgical treatment for an intramedullary spinal cord tumor from 2009 to 2020 in 58 facilities across Japan [34].
For this subanalysis, we retrospectively reviewed the data of 26 patients in the NSJ study who were pathologically diagnosed with subependymoma. Clinical presentation, duration of symptoms, magnetic resonance imaging (MRI) findings, neurological function before surgery, extent of surgical resection, diagnostic accuracy of intraoperative frozen pathology, adjuvant treatment, postoperative neurological function, tumor recurrence or progression, and surgical complications were recorded. Neurological function was assessed using the modified McCormick scale before surgery, immediately after, 6 months after, and at last follow-up (Table 1). The following MRI tumor characteristics were recorded: location and length, signal intensity, and enhancement pattern. Presence of tumor calcification on computed tomography was also recorded.
All tumors were resected via laminectomy using intraoperative neurophysiological monitoring. Surgery was performed by 17 surgeons certified by the NSJ from 17 institutions in Japan. Extent of resection was determined by each surgeon and classified as gross total resection (GTR), subtotal resection (STR; defined as ≥95% resection), partial resection (PR; defined as < 95% resection), or biopsy (Remove only a piece of tissue for pathological diagnosis). Tumor recurrence was defined as regrowth of the tumor on MRI after GTR. Tumor progression was defined in patients who underwent STR, PR, or biopsy as regrowth on MRI in conjunction with clinical deterioration.
Categorical variables are presented as numbers with percentage and were compared using the chi-square test or Fisher exact test as appropriate; multiple comparisons were performed after factor analysis using the chi-square test. Continuous variables are expressed as means with standard deviation or medians with interquartile range (IQR) and were compared using the t-test or Wilcoxon rank-sum test as appropriate; multiple comparisons were performed after factor analysis using analysis of variance or the Kruskal-Wallis test as appropriate. Statistical analyses were conducted using JMP Pro 16.0 software (SAS Institute Inc., Cary, NC, USA). All test were two-tailed. A p-value of < 0.05 was considered significant.
Patient characteristics are shown in Table 2. Seventeen patients were men and 9 were women. Median age was 49.4 years (range, 21–75 years). Median duration of symptoms was 24 months (IQR, 8.25–120 months). The most common symptoms were numbness in 22 patients (84.6%), motor weakness in 18 (69.2%), gait disturbance in 16 (61.5%), and pain in 13 (50%). Seven patients (26.9%) reported back pain and 7 reported bladder and/ or bowel dysfunction. Modified McCormick grade at presentation was as follows: grade I, 5 patients; grade II, 9 patients; grade III, 9 patients; and grade IV, 3 patients. Median follow-up was 40.5 months (IQR, 18–68 months).
Radiological findings are summarized in Table 3. Tumor location was cervical spine in 10 patients, cervicothoracic in 6, and thoracic in 10. Median tumor length was 60.5 mm (IQR, 26.8–101.8 mm). The tumor was located centrally in the spinal cord in 7 patients (26.9%) and eccentrically in 19 (73.1%). Seventeen tumors were unilateral (65.3%). Tumor signal was iso-to hypointense on T1-weighted imaging (T1WI); 18 tumors (69.2%) were isointense. On T2-weighted imaging (T2WI), the tumor signal was iso- to hyperintense; 24 tumors (92.3%) were hyperintense. Gadolinium enhancement was poor in 13 tumors (50%) and partial or slight in 13 (50%). Tumor consistency based on MRI was solid in 18, mixed in 7, and cystic in 1. Only 15 tumors (57.7%) were diagnosed as subependymoma before surgery. Three of the 7 centrally located tumors (42.9%) and 12 of the 17 unilateral tumors (70.6%) were correctly diagnosed as subependymoma before surgery. Among the 11 tumors that were not correctly diagnosed before surgery, 4 were diagnosed as astrocytoma and 2 as ependymoma.
Median surgeon experience was 30 years (IQR, 23–34.3 years). Extent of resection was GTR in 6 patients (23.1%), STR in 9, PR in 9, and biopsy in 2.
Fig. 1 shows time course of modified McCormick scale by extent of resection. Modified McCormick grade improved after surgery in 2 patients who underwent GTR. Postoperative worsening occurred in 6 patients overall (23.1%). By extent of resection, postoperative worsening occurred in 2 of the 6 patients who underwent GTR (33.3%), 3 of the 9 who underwent STR (33.3%), and 1 of 2 who underwent biopsy. All 9 patients who underwent PR experienced no postoperative neurological deterioration. The only postoperative complication was a single case of cerebrospinal fluid leakage. No patient received additional radiotherapy for residual tumor. Compared with that before surgery, the modified McCormick grade 6 months after surgery improved in 4 patients (2 of the 9 patients who underwent PR and 2 of the 6 patients who underwent GTR) and worsened in 5 (1 of the 2 who underwent biopsy, 1 of the 9 who underwent PR, 2 of the 9 who underwent STR, and 1 of the 6 who underwent GTR).
Compared with that 6 months after surgery, the modified McCormick scale at last follow-up had improved in 4 patients, worsened in 5, and remained unchanged in 17. Neurological outcome 6 months after surgery did not significantly differ based on neurological function before surgery (modified McCormick grade ≤ 2 vs. grade ≥ 3), tumor size (< 3 cm vs. ≥ 3 cm), location (cervical vs. other), nor extent of surgical resection (GTR vs. other). However, the proportion of patients who experienced neurological improvement 6 months after surgery was significantly higher in patients with preoperative bladder and/or bowel dysfunction than in those who did not (42.9% vs. 5.3%; crude odds ratio, 13.5; 95% confidence interval, 1.1–166, p = 0.047) (Table 4).
Three patients required multiple surgeries. Case 2 initially underwent biopsy and laminoplasty but the residual tumor grew 90 months later and was partially resected. Case 3 had a tumor extending from the pons to T5 and resection was performed in 2 stages 4 months apart. In case 8, neurological function remained stable after PR of a tumor extending from C5 to T5; reoperation was required 5.5 years later because of progression.
Intraoperative frozen-section pathology was performed in 18 cases (69.2%). The frozen section and final pathological diagnoses agreed in 9 (50%). Among the 9 cases in which these diagnoses differed, the frozen-section diagnosis was nonspecific (e.g., low-grade glioma) in 8 and 1 was incorrectly diagnosed as ependymoma. The Ki-67 labeling index was measured in 22 tumors and was less than 1% in 18.
A 40-year-old woman presented with a 1-month history of progressive back pain and bilateral leg numbness. Neurological examination showed 4−/5 strength on manual muscle testing in both lower extremities and bladder and bowel dysfunction. Modified McCormick grade was IV. MRI revealed a centrally located intramedullary mass lesion with 63 mm long in the spinal cord extending from T12 to L1. The lesion was hypointense on T1WI and hyperintense on T2WI. An area of focal enhancement was noted in the cranial portion (Fig. 2). The patient underwent T12 and L1 laminectomies for resection using a midline approach. The tumor contained cystic components and extended posteriorly and laterally from the central canal. The dissection plane was relatively clear. The intraoperative frozen pathology diagnosis was pilocytic astrocytoma. After the operation, her paraplegia was relieved but the sensory disturbance persisted; she was able to walk independently. The final histopathological diagnosis was subependymoma. In over 9 years of follow-up, she has remained recurrence-free and her neurological function has remained modified McCormick grade II.
A 48-year-old man presented with a 10-year history of low back pain followed by numbness of the right leg. Six months being referred to our hospital, the patient developed neck pain, numbness and muscle weakness of the left arm. MRI showed an eccentric intramedullary mass extending longitudinally from C2 to C6 with 80 mm long. The lesion was isointense on T1WI and hyperintense on T2WI with poor gadolinium enhancement (Fig. 3). Neurological examination showed muscle weakness involving the left arm and leg. Modified McCormick scale was II. The patient was diagnosed with subependymoma based on the gradually progressive clinical course and the specific radiological findings.
After C1 laminectomy and C2–7 laminotomy, the tumor was removed in a piecemeal fashion via the left dorsal root entry zone under motor evoked potential monitoring. The solid tumor extended from the central to the left lateral side. The tumor was elastic and demarcated; however, multiple small feeders from the pia mater precluded GTR. After maximal resection while preserving neurological function under monitoring, the surgery was aborted in STR because the intraoperative frozensection diagnosis was subependymoma. Postoperatively, the patient developed weakness in the left deltoid and biceps muscles and thermohypesthesia in the trunk on the right side. The left deltoid muscle weakness and thermohypesthesia partially recovered but the biceps muscle weakness did not. The permanent section diagnosis was subependymoma. In the 127 months of follow-up, there has been no tumor progression and the patient has remained independent with modified McCormick scale II.
Central nervous system subependymoma accounts for less than 1% of all intracranial tumors [9,35]. Spinal cord subependymoma is even rarer—only 113 surgical cases have been reported to date in numerous case reports and series [1,5-31]. Subependymomas are more common in middle-aged men in the fourth decade of life (mean age, 46 years) and frequently occur in the fourth and lateral ventricles [1]. In 2 recent case series of spinal cord subependymoma [5,6], most patients were men in their 40s and cervical and cervicothoracic sites were most common. In this series of 26 subependymoma patients from the 2022 NSJ study of intramedullary spinal cord tumors, the male-to-female ratio was 1.9:1, mean age was 49.4 years, and 65.4% of tumors were located in the cervical or cervicothoracic cord, consistent with previous reports. The corresponding values for patients with spinal ependymoma (1.2:1, 50.9 years, and 69.5%, respectively) and spinal astrocytoma (1.5:1, 43.9 years, and 55.2%, respectively) in the NSJ study were similar. However, mean duration of symptoms was significantly longer in subependymoma patients (57.7 months) than patients with ependymoma (25.2 months, p<0.001) or astrocytoma (14.9 months, p<0.001). Analysis of our study indicated that subependymoma is the slowest growing among all intramedullary spinal cord gliomas.
In the patients of this series, numbness in the extremities was the most frequent symptom (84.6%), followed by motor paralysis (69.2%), and gait disturbance (61.5%), which is consistent with previous case series [1,5,6]. However, modified McCormick grade before surgery was I or II in almost all patients in 2 of these series [5,6]; in contrast, modified McCormick grade was I or II in slightly more than half of patients in this series.
An accurate preoperative diagnosis based on MRI findings and clinical course is helpful when selecting the surgical strategy for treatment of an intramedullary spinal cord tumor. Spinal cord subependymoma is iso- to hypointense on T1WI, hyperintense on T2WI, and poorly or partially enhances [1,5,6,13,30]. Because these findings are similar to those of ependymoma, the 2 are difficult to distinguish [36]. However, subependymoma is frequently located eccentrically and rarely calcifies, which can aid with differentiation [1,6]. In this series, 18 subependymomas (69.2%) were isointense on T1WI, 24 (92.3%) were hyperintense on T2WI, and half poorly enhanced. Furthermore, location was eccentric in 19 (73.0%), and calcifications were present in only 3 of the 23 cases (13.0%) in which computed tomography was performed. Despite these characteristic findings, only 57.7% were correctly diagnosed with subependymoma before surgery. Diagnostic accuracy was 63.2% in tumors with eccentric location and 42.9% in those located centrally. The low accuracy may be explained by the rarity of subependymoma and the fact that other intramedullary lesions appear similar on MRI. To improve diagnostic accuracy for subependymoma, clinicians should consider both clinical course and lesion location.
Subependymomas are histologically benign and classified as World Health Organization grade 1. Histological features that distinguish it from ependymoma or astrocytoma are: (1) well-demarcated and multinodular mass; (2) low or moderate cellularity; (3) microlobular pattern; and (4) small clusters of neoplastic cells [7]. Ependymomas are classified as grade 2 and characterized by perivascular pseudorosettes with long fibrillary processes [31,37]. Because the extent of surgical resection is associated with progression-free survival in patients with ependymoma [31], accurate intraoperative diagnosis with frozen pathology is ideal. Previously reported accuracy rates of intraoperative diagnosis for spinal cord ependymoma and astrocytoma are 72% and 71%, respectively, which are considerably lower than those for brain tumors (83%–97%) [37]. In this study, the accuracy rate was only 50%, which is even lower. One tumor was incorrectly diagnosed as ependymoma and 8 were rendered a nonspecific diagnosis, such as low-grade glioma. Possible reasons for the low accuracy rates include frozen-section artifact, small sample size, and limited pathologist experience with subependymoma [7]. In this study, GTR was achieved in only 22.2% of the tumors correctly diagnosed as subependymoma during surgery. The reasons for the low GTR rate include poorly defined tumor-spinal cord interface and tumor location near the pyramidal tract. Surgeons should consider that the accuracy of intraoperative diagnosis for intramedullary spinal cord tumors is low when selecting surgical strategy.
Surgical excision of subependymoma is expected to result in good clinical outcomes with low risk of recurrence or progression. Radiation is not recommended as adjuvant therapy [1]. The basic surgical strategy for spinal cord subependymoma is to resect as much tumor as possible [5,8,27]. In a series of 6 patients with spinal cord subependymoma in whom GTR was achieved, Jallo et al. [8] reported neurological deterioration in all; however, 5 recovered within 6 months. They recommend aggressive surgical excision because GTR is expected to be curative. However, Yuh et al. [6] reported their experience with 10 surgical cases and noted that the tumor did not always detach easily from the spinal cord and only half could be completely resected. Because subependymoma is an indolent tumor, they recommended that preservation of neurological function should be prioritized and that STR is a reasonable option. In this series of subependymoma patients from the 2022 NSJ study, GTR was achieved in only 23.1%, which was higher than 10.7% in astrocytoma patients but significantly lower than the 74.8% rate achieved in the ependymoma patients [34]. Among the 6 GTR cases, the tumor was centrally located in 3. Only 3 of the 19 eccentrically located tumors were completely resected. As described above, the tumor in case 24 progressed from the central canal toward the posterior columns with minimal invasion of the anterior and lateral columns and was completely resected. In addition, the tumor’s cystic component and clear demarcation from the spinal cord were factors that contributed to the favorable outcome. In contrast, in case 19, the longitudinally extensive eccentric tumor with multiple small feeders precluded GTR; therefore, the resection was limited to preserve neurological function. One of the probable reasons for the low spinal subependymoma GTR rate is tumor location or extension adjacent to the pyramidal tract. The extent of resection must be restricted to preserve neurological function.
Neurological function before surgery, tumor extension, and extent of resection are factors reportedly associated with functional outcome in patients with spinal ependymoma [31,38-40]. In this study, preoperative neurological function and tumor extension were not associated with better functional outcome 6 months after surgery; however, preoperative bladder and/or bowel dysfunction was. It is not possible to clarify why the presence of preoperative bladder and bowel dysfunction is associated with favorable surgical outcomes, as multiple factors may be involved. One speculation is that cases with bladder and bowel dysfunction in this series were younger (42.7 years vs. 51.9 years) and had shorter duration of symptoms (47 months vs. 61.7 months) than those without bladder and bowel dysfunction, which may have contributed to postoperative good recovery. Based on the results of our study, early surgical excision is recommended for spinal subependymoma cases with bladder and bowel dysfunction because functional improvement can be expected with surgery.
In this series, recurrence did not occur in any of the patients in whom GTR was achieved (mean follow-up, 60±37.3 months), indicating that resection is curative. In 2 patients who underwent PR and biopsy, respectively, progression occurred after 68 and 90 months, respectively, and re-resection was indicated. Both patients recovered to their preoperative state. Five patients experienced neurological worsening after surgery and 4 of these had improved at last follow-up; however, functional outcome did not significantly differ between GTR and lesser extents of resection. Favorable long-term outcome after STR has also been reported for spinal subependymoma in another study [5]. We recommend aiming for GTR but not at the cost of causing loss of neurological function, which should be monitored electrophysiologically during surgery. For tumors with a poorly defined tumor-spinal cord interface, STR or less is acceptable to avoid the risk of causing a neurological deficit. Because radiotherapy is not recommended for residual subependymoma, careful followup is necessary after partial removal. A second surgery should be considered if progression occurs.
The major limitations of this study include its small sample size and relatively short follow-up period. In addition, because it was retrospective in design and included data from patients treated in 58 centers, the experience and skill of the numerous radiologists, pathologists, and surgeons involved was variable. Furthermore, the particular experience of these individual physicians and surgeons with spinal cord subependymoma is limited because it is so rare. Because this study comprises the largest case series reported to date and all patients were diagnosed and treated by experienced surgeons certified by the NSJ, we are convinced that it will be useful for guiding diagnosis and treatment of patients with spinal subependymoma.
Spinal cord subependymoma is an indolent tumor which is frequently located eccentrically, a characteristic that is useful to differentiate it from other intramedullary spinal cord tumors. Although GTR is curative and should be the aim of surgical treatment, many subependymomas are not completely resectable without causing a neurological deficit. PR to preserve neurological function is acceptable in cases in which the tumor-spinal cord interface is poorly defined.
NOTES
Author Contribution
Conceptualization: TY, MM, TE; Data curation: TY, TE, YT, MI, RK, KT, MN, KH; Formal analysis: TY, HK, KT; Funding acquisition: MM; Methodology: TY, MM, HK, TE; Project administration: MM, TE; Visualization: TY; Writing - original draft: TY; Writing - review & editing: MM, HK, KT, TE, RK, KT, MN, KH.
ACKNOWLEDGEMENTS
Portions of this work were presented in abstract at the thirty-sixth annual meeting of the Neurospinal Society of Japan (2021).
Participating institutions of the NSJ study are: Aichi Medical University, Akita Cerebrospinal and Cardiovascular Center, Akita University, Iwakuni Clinical Center, Iwate Medical University, Utsunomiya Brain and spinal cord center, Ehime University. Osaka City General Hospital, Osaka City University. Ohnishi Neurological Center, Okayama University. Otaru City Hospital, Kawasaki Medical School, Kitano Hospital. Gifu University, Kyoto Prefectural University of Medicine, Kushiro Kojinkai Memorial Hospital, Spine and Low Back Pain Center, Kitasuma Hospital, Kurume University, Kochi University, Kobe University, Saitama Medical University, International Medical Center, Sapporo Azabu Neurosurgical Hospital, Sapporo Medical University, Juntendo University, Shonan Kamakura General Hospital, Yokkaichi Municipal Hospital, Shin Komonji Hospital, Shinyurigaoka General Hospital, Seirei Hamamatsu General Hospital, Tokyo General Hospital, Southern Tohoku Research Institute for Neuroscience, Tohoku University, Tokyo University, Tokyo Medical and Dental University, The Jikei University School of Medicine, Toho University, Dokkyo Medical University, Tomakomai City Hospital. Tottori University, Tokyo Metropolitan Neurological Hospital, Nakamura Memorial Hospital, Nara Medical University, Niigata City hospital, Nippon Medical School, Brain Attack Center, Ota Memorial Hospital, Hyogo Medical University, Hiroshima University, Fukushima Medical University, Fujieda Heisei Memorial Hospital, Fujita Health University, Hokkaido University, Hokkaido Neurosurgical Memorial Hospital, Hokkaido Medical Center for Child Health and Rehabilitation, Mie University, Moriguchi-Ikuno Memorial Hospital, University of Yamanashi, Yokohama City University, Wakayama Medical University.
The authors thank the Neurospinal Society of Japan investigators of intramedullary spinal cord tumors: Masahito Hara and Masahiro Aoyama of Aichi Medical University; Kazushige Itoki of Utsunomiya Brain and Spinal Cord Center; Takao Yasuhara of Okayama University; Hideki Hayashi of Kitano Hospital; Syunsuke Yano of Sapporo Azabu Neurosurgical Hospital; Hidetoshi Murata of St.Marianna University of Medicine; Hiroki Ohashi of Jikei University School of Medicine; Yasufumi Ohtake of Nakamura Memorial Hospital; Akihiko Saito of Niigata City Hospital; Jun Muto and Tatsushi Inoue of Fujita Health University; Kazuyoshi Yamazaki of Hokkaido University; Izumi Koyanagi of Hokkaido Neurosurgical Memorial Hospital; and Masashi Fujimoto of Mie University.
Fig. 1.
Time course of modified McCormick scale by extent of resection. Patients who achieved GTR had a higher preoperative rate of good neurological function, while those who remained in PR had a higher rate of severe neurological function preoperatively. The functional prognosis of the GTR group was good, and neurological function tended to be maintained even with resection less than STR. GTR, gross total resection; STR, subtotal resection; PR, partial resection; Preop, preoperative; op, operation; 6M, 6 months; F/U, follow-up.
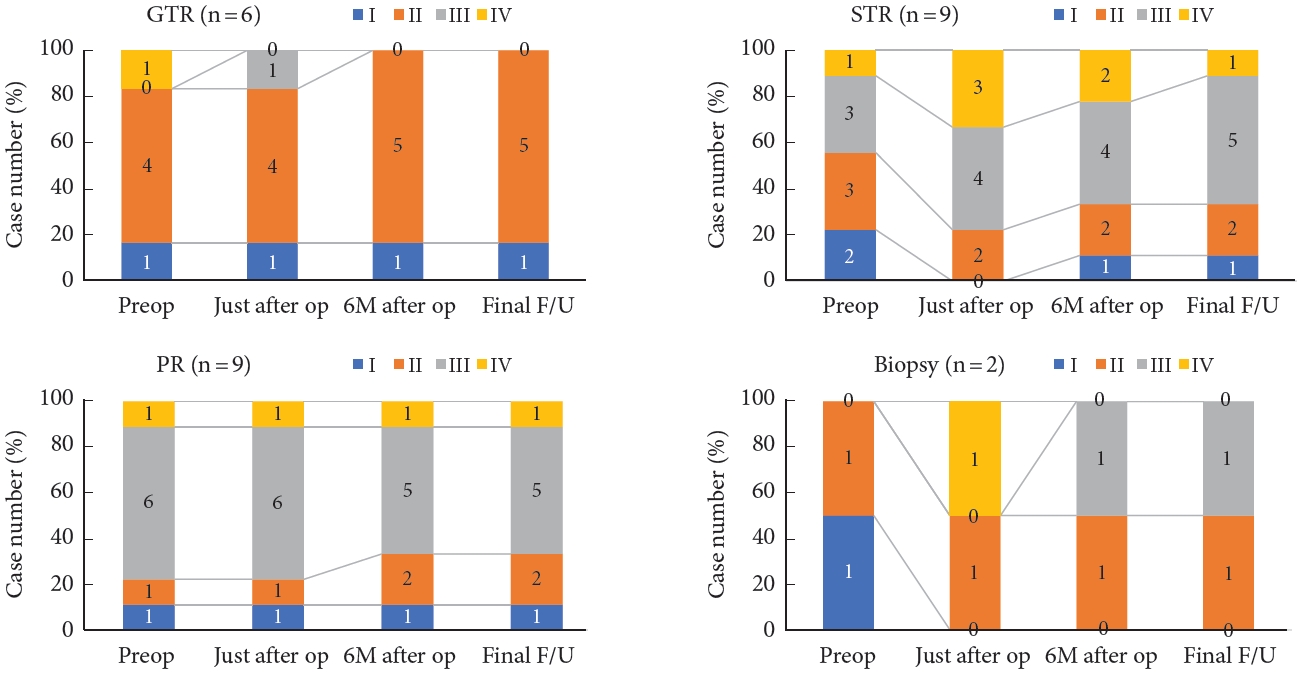
Fig. 2.
Preoperative magnetic resonance imaging in case 24 showed an intramedullary mass with cystic component at T12-L1. The mass was hypointense on T1-weighted images (A) and exhibited partial enhancement (B). (C) It was hyperintense on T2- weighted images. (D) Axial T2-weighted images showed a centrally located intramedullary cystic mass. (E) Sagittal T2-weighted imaging 6 months after surgery demonstrated complete removal.
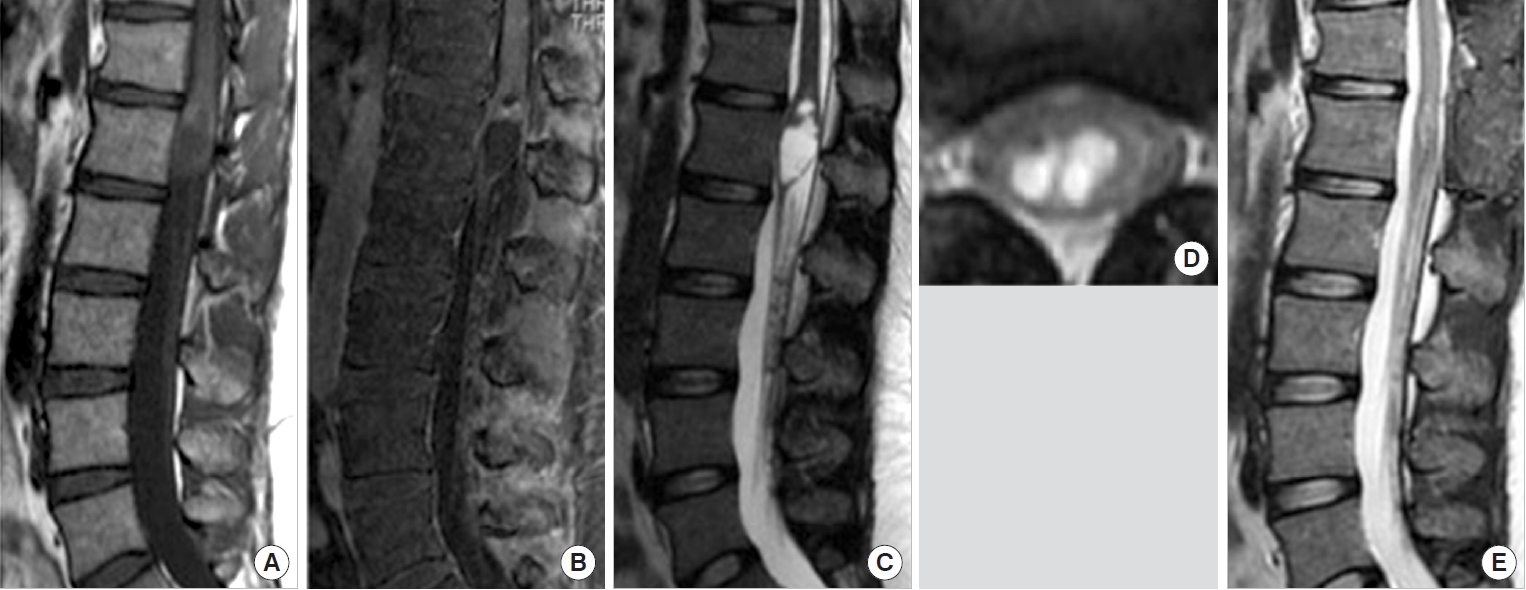
Fig. 3.
Preoperative magnetic resonance imaging in case 19 showed an intramedullary mass located at C2–6. The mass was isointense on T1- weighted images (A) and exhibited partial weak enhancement (B). (C) It was hyperintense on T2-weighted images. Coronal (D) and Axial (E) T2-weighted images showed the mass was located eccentrically on the left. Sagittal (F) and coronal (G) T2-weighted images 1 month after surgery showed subtotal resection.
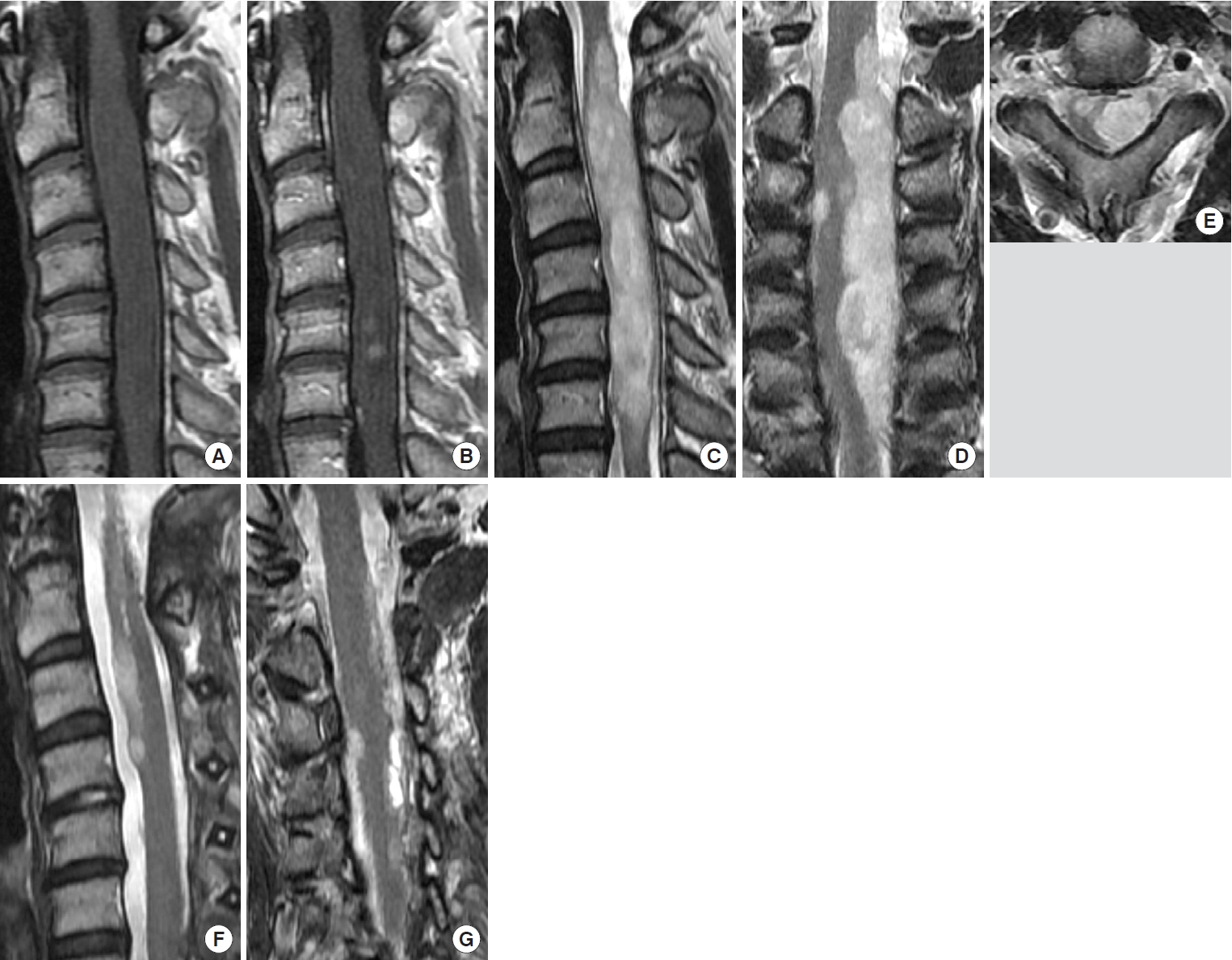
Table 1.
Modified McCormick scale
Table 2.
Summary of demographics and clinical presentations of 26 patients
Table 3.
Summary of radiological findings of 26 patients
Table 4.
Prognostic factors for neurological function 6 months after surgery
| Variable |
Subependymoma |
p-value | ||||
|---|---|---|---|---|---|---|
| All (n = 26) | Improved (n = 4) | Stable (n = 17) | Worsened (n = 5) | |||
| Preoperative neurological findings | 0.26 | |||||
| Modified MS grade I/II | 14 (53.9) | 1 (25) | 9 (52.9) | 4 (80) | ||
| Modified MS grade III/IV/V | 12 (46.2) | 3 (75) | 8 (47.1) | 1 (20) | ||
| Tumor size (cm) | 0.52 | |||||
| < 3 | 7 (26.9) | 2 (50) | 4 (23.5) | 1 (20) | ||
| ≥3 | 19 (73.1) | 2 (50) | 13 (76.5) | 4 (80) | ||
| Location | 0.46 | |||||
| Cervical lesion | 10 (38.5) | 1 (25) | 8 (47.1) | 1 (20) | ||
| Noncervical lesion | 16 (61.5) | 3 (75) | 9 (52.9) | 4 (80) | ||
| The extent of surgical resection | 0.38 | |||||
| GTR | 6 (23.1) | 2 (50) | 3 (17.7) | 1 (20) | ||
| Non-GTR | 20 (76.9) | 2 (50) | 14 (82.4) | 4 (80) | ||
| Preoperative appearance of bladder and bowel dysfunction | 0.04** | |||||
| Bladder and bowel dysfunction | 7 (26.9) | 3 (75) | 4 (23.5) | 0 | ||
| Nonbladder and bowel dysfunction | 19 (73.1) | 1 (25) | 13 (76.5) | 5 | ||
REFERENCES
1. Rincon-Torroella J, Rakovec M, Khalafallah AM, et al. Clinical features and surgical outcomes of intracranial and spinal cord subependymomas. J Neurosurg 2022 Feb 11:1-12. doi: 10.3171/2021.12.JNS211643. [Epub].

2. Rushing EJ, Cooper PB, Quezado M, et al. Subependymoma revisited: clinicopathological evaluation of 83 cases. J Neurooncol 2007;85:297-305.



3. Scheithauer BW. Symptomatic subependymoma. Report of 21 cases with review of the literature. J Neurosurg 1978;49:689-96.

4. Ragel BT, Osborn AG, Whang K, et al. Subependymomas: an analysis of clinical and imaging features. Neurosurgery 2006;58:881-90. discussion 881-90.



5. Wu L, Yang T, Deng X, et al. Surgical outcomes in spinal cord subependymomas: an institutional experience. J Neurooncol 2014;116:99-106.



6. Yuh WT, Chung CK, Park SH, et al. Spinal cord subependymoma surgery: a multi-institutional experience. J Korean Neurosurg Soc 2018;61:233-42.




7. Choi SK, Lee SH, Kim B, et al. Findings from frozen sections of spinal subependymomas: is it possible to differentiate this diagnosis from other common spinal tumors? Brain Tumor Pathol 2016;33:19-26.



8. Jallo GI, Zagzag D, Epstein F. Intramedullary subependymoma of the spinal cord. Neurosurgery 1996;38:251-7.


9. Jain A, Amin AG, Jain P, et al. Subependymoma: clinical features and surgical outcomes. Neurol Res 2012;34:677-84.



10. D’Amico RS, Praver M, Zanazzi GJ, et al. Subependymomas are low-grade heterogeneous glial neoplasms defined by subventricular zone lineage markers. World Neurosurg 2017;107:451-63.


11. Wu Z, Iwanami A, Yasuda A, et al. Intramedullary cervicothoracic subependymoma: report of three cases and review of the literature. J Orthop Sci 2015;20:927-34.


12. Wu L, Tian Y, Wang L, et al. Subependymoma of the conus medullaris with cystic formation: case report and a literature review. World Neurosurg 2020;137:235-8.


13. Hoeffel C, Boukobza M, Polivka M, et al. MR manifestations of subependymomas. AJNR Am J Neuroradiol 1995;16:2121-9.


14. Artico M, Bardella L, Ciappetta P, et al. Surgical treatment of subependymomas of the central nervous system. Report of 8 cases and review of the literature. Acta Neurochir (Wien) 1989;98:25-31.

15. Shimada S, Ishizawa K, Horiguchi H, et al. Subependymoma of the spinal cord and review of the literature. Pathol Int 2003;53:169-73.


16. Orakcioglu B, Schramm P, Kohlhof P, et al. Characteristics of thoracolumbar intramedullary subependymomas. J Neurosurg Spine 2009;10:54-9.


17. Nakasu S, Nakasu Y, Saito A, et al. Intramedullary subependymoma with neurofibromatosis--report of two cases. Neurol Med Chir (Tokyo) 1992;32:275-80.


18. Lee KS, Angelo JN, McWhorter JM, et al. Symptomatic subependymoma of the cervical spinal cord. Report of two cases. J Neurosurg 1987;67:128-31.

19. Takami T, Yamagata T, Ohata K. Posterolateral sulcus approach for spinal intramedullary tumor of lateral location: technical note. Neurol Med Chir (Tokyo) 2013;53:920-7.



20. Iwasaki M, Hida K, Aoyama T, et al. Thoracolumbar intramedullary subependymoma with multiple cystic formation: a case report and review. Eur Spine J 2013;22 Suppl 3(Suppl 3):S317-20.



21. Matsumura A, Hori A, Spoerri O. Spinal subependymoma presenting as an extramedullary tumor: case report. Neurosurgery 1988;23:115-7.



22. Guha A, Resch L, Tator CH. Subependymoma of the thoracolumbar cord. Case report. J Neurosurg 1989;71:781-7.

24. Nishimura H, Fukami S, Endo K, et al. A case of rapidly-progressing cervical spine subependymoma with atypical features. Spine Surg Relat Res 2019;3:91-4.


25. Oishi M, Fujisawa H, Tsuchiya K, et al. Spinal cord subependymoma mimicking syringomyelia in a child: a case report. Childs Nerv Syst 2021;37:2667-71.



26. Matsumoto K, Nakagaki H. Intramedullary subependymoma occupying the right half of the thoracic spinal cord--case report. Neurol Med Chir (Tokyo) 2002;42:349-53.


27. Bret P, Bougeard R, Saint-Pierre G, et al. Sub-épendymome médullaire cervical. Revue de la littérature à propos d’une observation [Intramedullary subependymoma of the cervical spinal cord. Review of the literature a propos of a case]. Neurochirurgie 1997;43:158-63.

28. Tan LA, Kasliwal MK, Mhanna N, et al. Surgical resection of subependymoma of the cervical spinal cord. Neurosurg Focus 2014;37 Suppl 2:Video 3.


29. Cure LM, Hancock CR, Barrocas AM, et al. Interesting case of subependymoma of the spinal cord. Spine J 2014;14:e9-12.

30. Zhou S, Xiong J, Pan J, et al. Neuroradiological features of cervical and cervicothoracic intraspinal subependymomas: a study of five cases. Clin Radiol 2016;71:499.e9-15.


31. Bostrom A, von Lehe M, Hartmann W, et al. Surgery for spinal cord ependymomas: outcome and prognostic factors. Neurosurgery 2011;68:302-8. discussion 9.



32. Boykin FC, Cowen D, Iannucci CA, et al. Subependymal glomerate astrocytomas. J Neuropathol Exp Neurol 1954;13:30-49.

33. Soleiman HA, Ironside J, Kealey S, et al. Spinal subependymoma surgery: do no harm. Little may be more! Neurosurg Rev 2020;43:1047-53.



34. Endo T, Inoue T, Mizuno M, et al. Current trends in the surgical management of intramedullary tumors: a multicenter study of 1,033 patients by the Neurospinal Society of Japan. Neurospine 2022;19:441-52.




35. Jabri HE, Dababo MA, Alkhani AM. Subependymoma of the spine. Neurosciences (Riyadh) 2010;15:126-8.

36. Kobayashi K, Ando K, Kato F, et al. MRI characteristics of spinal ependymoma in WHO grade II: a review of 59 cases. Spine (Phila Pa 1976) 2018;43:E525-30.

37. Hongo H, Takai K, Komori T, et al. Intramedullary spinal cord ependymoma and astrocytoma: intraoperative frozensection diagnosis, extent of resection, and outcomes. J Neurosurg Spine 2018;30:133-9.


38. Baig Mirza A, Gebreyohanes A, Knight J, et al. Prognostic factors for surgically managed intramedullary spinal cord tumours: a single-centre case series. Acta Neurochir (Wien) 2022;164:2605-22.




- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 2,397 View
- 165 Download
-
Journal Impact Factor 3.8
SURGERY: Q1
CLINICAL NEUROLOGY: Q1






























