 |
 |
- Search
| Neurospine > Volume 20(3); 2023 > Article |
|
|
Abstract
Objective
The characteristics, imaging features, long-term surgical outcomes, and recurrence rates of primary spinal pilocytic astrocytomas (PAs) have not been clarified owing to their rarity and limited reports. Thus, this study aimed to analyze the clinical presentation, radiological features, pathological findings, and long-term outcomes of spinal PAs.
Methods
Eighteen patients with spinal PAs who were surgically treated between 2009 and 2020 at 58 institutions were included in this retrospective multicenter study. Patient data, including demographics, radiographic features, treatment modalities, and long-term outcomes, were evaluated.
Results
Among the 18 consecutive patients identified, 11 were women and 7 were men; the mean age at presentation was 31 years (3–73 years). Most PAs were located eccentrically, were solid or heterogeneous in appearance (cystic and solid), and had unclear margins. Gross total resection (GTR), subtotal resection (STR), partial resection (PR), and biopsy were performed in 28%, 33%, 33%, and 5% of cases, respectively. During a follow-up period of 65 ± 49 months, 4 patients developed a recurrence; however, the recurrence-free survival did not differ significantly between the GTR and non-GTR (STR, PR, and biopsy) groups.
Conclusion
Primary spinal PAs are rare and present as eccentric and intermixed cystic and solid intramedullary cervical tumors. The imaging features of spinal PAs are nonspecific, and a definitive diagnosis requires pathological support. Surgical resection with prevention of neurological deterioration can serve as the first-line treatment; however, the resection rate does not affect recurrence-free survival. Investigation of relevant molecular biomarkers is required to elucidate the regrowth risk and prognostic factors.
Pilocytic astrocytomas (PAs) are World Health Organization (WHO) grade I tumors that account for approximately 25% and 1.5% of all pediatric and adult brain tumors, respectively [1,2]. They can occur anywhere in the neuraxis, with the cerebellum, cerebral hemispheres, optic tract, and hypothalamus and the brainstem being the most common locations [1,3]. They are generally considered to be slow-growing, well-defined lesions that are surgically curable with gross total resection (GTR) [4,5]. Primary spinal PAs constitue a rare subset of these tumors, accounting for only 2% of all PAs and 21% of all intramedullary glial tumors in pediatric and young adult populations [6]. They include secondary spinal PAs caused by the dissemination of intracranial PAs through the cerebrospinal fluid [7,8]. Similar to other intramedullary spinal tumors, primary spinal PAs are managed using surgical resection [9]; however, clear evidence that supports the need for postoperative adjuvant treatment is lacking. Accurate preoperative diagnosis of spinal PAs helps in the planning of clinical treatments; surgeons can determine the optimal extent of resection and postoperative adjuvant treatments. Due to the rarity of the condition, less than 100 cases have been reported so far [10-21]. The common clinical data of such cases, such as demographics, natural history, optimal treatment, and prognosis, remain unclear [7,21-24].
Thus, the aim of this study was to analyze the clinical presentation, radiological features, and long-term outcomes of spinal PAs. Accordingly, we retrospectively analyzed the medical records and long-term follow-up data of 18 patients with spinal PAs in Japanese spinal tumor groups and reviewed the existing literature on the condition.
This study analyzed data obtained in a previous multicenter cohort study on patients treated between 2009 and 2020, which was authorized by the Neurospinal Society of Japan. This database collects data on the clinical courses and surgical outcomes of patients with intramedullary spinal cord tumors treated across 58 neurosurgical centers in Japan (Tables 1, 2) [25].
Eighteen patients with intramedullary spinal cord PAs who were treated surgically and diagnosed pathologically were included in a previous multicenter cohort study. Although the updated 2021 WHO classification specifies various genes, molecules, pathways, and histological findings for diagnosis, patient diagnoses in this study were based on conventional histopathological grading. The extent of resection was classified as macroscopic GTR (100%), subtotal resection (STR; >90%), partial resection (PR; <90%), or an open biopsy.
The modified McCormick scale (MMCS) was used to assess the neurological function both perioperatively and during follow-up [26]. We extracted the following data from the database; these data were anonymized: age; sex; duration of symptoms, presence of intracranial lesions; initial diagnosis; resection rate; follow-up term; term of recurrence; MMCS grades preoperatively, postoperatively, at the 12-month follow-up, and at the final follow-up; magnetic resonance imaging (MRI) data, including T1-weighted imaging (WI) findings, T2WI findings, gadolinium (Gd)-enhanced findings, lesion boundary and distribution, syringomyelia, lesion shape and appearance, vertical segment, and maximal length (mm) in the sagittal plane; pathological diagnoses; and histopathological diagnoses. Patients with tumor relapse due to local recurrence/progression, diffuse meningeal spread, or both were categorized into the disease progression subgroup; those without a tumor relapse were categorized into the no-disease progression subgroup. The patients’ postoperative clinical performance status, with or without adjuvant radiotherapy, was compared between the 2 subgroups.
The survival period, defined as the number of months from surgery to death, was censored at the last available follow-up or the cutoff study date (December 31, 2020) for those who were still alive. The recurrence-free survival was estimated using the Kaplan-Meier method with associated log-rank tests for the entire cohort. Statistical analysis was performed using JMP ver. 14 (SAS Institute Inc., Cary, NC, USA). Binary variables were analyzed using the chi-square test. Continuous variables were compared between the subgroups using the Mann-Whitney U-test. A p-value of <0.05 was considered significant. Significance level was set at 5%.
Eighteen patients (comprising 11 women and 7 men) were included in this study. The overall mean age was 31.7 ±18.5 years (range, 3–73 years). At admission, neurological examination revealed the following: headache (n=3 [17%]), back pain (n=7 [39%]), extremity pain (n=3 [17%]), extremity weakness (n=13 [72%]), gait disturbance (n=13 [72%]), and bladder and rectal disturbances (n=5 [28%]). The symptoms lasted for varing durations, the mean duration was 7.5±11.2 months (range, 0–38 months). The preoperative MMCS grades at admission were I, II, III, and IV in 3 (17%), 8 (44%), 5 (28%), and 2 patients (11%), respectively (Table 1).
The average PA size was 66.1±54.5 mm (range, 13–178 mm); the average vertebral level involved was 4.2 (range, 1–13). Of the 18 patients included, 8 (44%), 3 (17%), and 7 patients (10%) had cervical, thoracic, and cervicothoracic junction tumors, respectively. During preoperative MRI, T1WI revealed low intensity and is intensity lesions in 42% and 58% of the patients, respectively. Conversely, T2WI revealed low intensity and high-intensity lesions in 17% and 83% of the patients, respectively. Gd-enhanced T1WI revealed heterogeneous, ring-shaped, and homogenous enhancements in 78%, 6%, and 6% of the patients, respectively; no enhancement was observed in 11% of the patients. The tumor locations were centric and eccentric in 33% and 67% of the patients, respectively; with the tumor borders being regular in 33% and irregular in 67%. The tumors were solid, cystic, and both in 50%, 11%, and 39% of the patients, respectively. Two spinal PAs (10%) showed evidence of hemorrhage. Additionally, 8 patients (44%) had a secondary syrinx: the cervivcal and thoracic regions were involed in 5 and 3 of these patients, respectively (Table 2).
All patients had PAs, and a total of 18 tumors were treated: GTR, STR, PR and biopsy were performed in 5 (28%), 6 (33%), 6 (33%), and 1 patient (6%), respectively. The mean MIB-1 labeling index was 2.8%. In terms of perioperative complications, no cerebrospinal fluid leakage, postoperative hemorrhage, or infection was observed in the surgical area in any patient.
Postoperatively, the MMCS grade improved in 6 patients (33%), remained unchanged in 10 patients (56%), and worsened in 2 patients (11%). Four patients experienced postoperative neurological deterioration: this was transient and normalized to the baseline status in 2 patients (cases #3 and #6) but permanent in the other 2 patients (cases #14 and #16).
The average follow-up period was of 65.2 ±49.2 months (range, 11–140 months). No deaths occurred, but 4 cases of re-enlargement (22%) were observed. These 4 patients received additional treatments.
In case #5, MRI at admission revealed a high-intensity lesion on T2WI (Fig. 1A) and a heterogeneously enhanced, unclear, and eccentrically shaped lesion on Gd-enhanced T1WI (Fig. 1B: sagittal; Fig. 1C: axial). The patient underwent a biopsy (Fig. 1D) and received radiotherapy combined with temozolomide (TMZ) chemotherapy, which resulted in a neurologically stable preoperative and postoperative MMCS grade of I. Fourteen months after the initial therapy, MRI revealed tumor regrowth on T2WI (Fig. 1E) and T1WI with Gd enhancement (Fig. 1F). The patient received TMZ+avastin monthly, and the tumor size was controlled for 13 months as seen on T2WI (Fig. 1G) and T1WI with Gd enhancement (Fig. 1H).
In case #13, preoperative MRI revealed a cystic, eccentric tumor with an irregular shape and an unclear boundary at the C1–2 level on T1WI (Fig. 1I) and T2WI (Fig. 1J) and on axial (Fig. 1K) and sagittal (Fig. 1L) Gd-enhanced T1WI. The initial surgery involved only partial removal of the tumor; 8 months later, MRI revealed tumor regrowth on T2WI (Fig. 1M) and sagittal T1WI with Gd enhancement (Fig. 1N). The patient received 12 courses of TMZ, and a second PR was performed 26 months after the initial surgery. After the second surgery, the patient received carboplatin chemotherapy and radiotherapy. The overall survival was of 122 months. MRI revealed the shrunken tumor on T2WI (Fig. 1O) and on T1WI with Gd enhancement (Fig. 1P).
In case #17, the patient underwent PR, received 12 cycles of TMZ at the outpatient clinic, and exhibited recurrence 134 months after the initial surgery. In case #18, the patient underwent GTR and no additional therapy; however, they experienced a recurrence 60 months later, and thus, underwent additional surgery and radiotherapy. The tumor did not recur until 140 months after treatment.
The tumor size (p=0.30) and extent of resection (p=0.64) did not play a role in recurrence. Furthermore, the recurrence-free survival did not differ significantly between the GTR and non-GTR (including biopsy, PR, and STR) groups (p=0.94) (Fig. 2).
Our study findings and literature review reveal that a preoperative imaging-based diagnosis of spinal PAs remains challenging. Following preoperative MRI, 11 out of 18 patients were radiologically diagnosed with astrocytomas (50%), ependymomas (22%), pilomyxoid astrocytomas (5%), and gliomas (11%). Astrocytomas and ependymomas are the most commonly misdiagnosed tumors. This misdiagnosis is attributed to the lack of distinctive imaging features; both spinal PAs and ependymomas present with secondary syringomyelia cavity formation and heterogeneous enhancement on imaging. In 4 cases in our series, the tumors mimicked ependymomas on MRI. However, spinal PAs usually show an eccentric growth pattern (67% in our case series), which is different from the characteristic centric growth pattern of ependymomas [20]. Spinal PAs are rarely associated with intratumoral hemorrhage; it occurred in only 10% of our cases, as compared to the incidence of 0.5% [17] and 12.5% [24] reported previously. Conversely, the incidence of intratumoral hemorrhage in spinal ependymomas is high at 78% [28]. Heterogeneous cyst formation, a common MRI feature of PAs reported previously, was observed in approximately two-thirds (67%) of the PAs in our study.
There were no characteristic findings of PAs on MRI. These tumors appeared solid (present study: 50%; literature: 30%–44%), cystic (present study: 11%), and both solid and cystic (present study: 39%; literature: 47%–70%). The tumor borders were clear (present study: 28%; literature: 15%–60%) and unclear (present study: 72%; literature: 40%–85%). The distributions were centric (present study: 33%; literature: 38.5%) and eccentric (present study: 67%; literature: 61.5%). Secondary syrinx was found in 44% of our patients (literature: 37%–55%). Two spinal PAs in our study (10%) showed evidence of hemorrhage (literature: 17%). On preoperative MRI, T1WI revealed lesions of low intensity (incidence in present study: 42%; literature: 10%–25%), isointensity (present study: 58%; literature: 42%:15%–69%), and high intensity (literature: 10%). On preoperative MRI, T2WI revealed lesions of low intensity (present study: 25%; literature: not available), high intensity (present study: 75%; literature: 63%–85%), mixed intensity (literature: 5%), and isointensity (literature: 10%–19%). Gd-enhanced T1WI revealed heterogeneous enhancement (present study: 78%; literature: 36%–45%), no enhancement (present study: 11%; literature: 0%–14%), homogenous enhancement (present study: 6%; literature: 21%–25%), and ring-shaped enhancement (present study: 6%; literature: 30%–42%).
Our findings suggest that the benefits and drawbacks of GTR do not play a role in tumor recurrence. The tumor size (p=0.30) and MIB-1 index (p=0.36) were also not associated with recurrence. Interestingly, MIB-1 index has been reported to be unrelated to prognosis. The MIB-1 index values in patients harboring with PA in our study were not significantly higher (mean, 2.7± 2.0) than those reported previously (≥ 4) in patients harboring with PAs of the brain [29]. None of the 18 patients in our study died, indicating no cases of mortality; however, 4 cases of recurrence were observed. The average term of observation was 65.2± 49.2 months (range, 11–140 months). Although complete surgical resection is considered the first-line treatment for spinal PAs to prevent the worsening of neurological symptoms [1,23], 5 out of 18 patients underwent total resection (28%) in our study with maintained or improved MMCS grades, and 4 experienced a recurrence. In recurrent cases, 1 case was biopsy, 2 cases were PR, and 1 was GTR. The purpose of surgery is to confirm the pathological characteristics, establish a future treatment plan, and decrease the tumor mass. In this study, 6 and 6 patients underwent STR and PR, respectively. The removal rate was not related to border irregularity (p=0.25), indicating that the tumor shape was not the only factor affecting the removal rate. Intraoperative neuromonitoring findings also played a role in determining the removal rate and postioperative neurological deficits. Some researchers consider cyst walls to represent tumor invasion [17], while others consider these walls to represent reactive changes [23,30]; our study found that aggressive removal of the cyst walls was unnecessary, as the removal rate did not contribute to recurrence.
Our study also demonstrated that functional recovery after a surgery for PA was challenging: only 6 out of the 18 patients (33%) analyzed showed an improvement, whereas the remaining 10 (56%) and 2 patients (11%) exhibited no changes and permanent deterioration in the MMCS grade, respectively. However, surgical treatment prevented both immediate and permanent worsening of the MMCS grade postoperatively (Table 3).
Four patients (STR, 1; PR, 2; Biopsy, 1) experienced tumor progression and required additional treatment, including surgery, chemotherapy, and radiotherapy. Various treatments have been administered for PA recurrence. Despite incomplete resections and no adjuvant therapy, the tumor remained stable in 14 patients (78%) without growth during an average follow-up period of 65.2 months. While a few reports have described the use of chemotherapy for spinal PAs [30], the efficacy of chemotherapy and radiation therapy for spinal PAs remains unclear [12,14,27,30]. Therefore, in the future, it will be necessary to verify the efficacy of chemoradiation therapy by analyzing not only histological data, but also molecular data.
A considerable number of reports are available on PAs [13,15-17,27]. We have compared our study findings with those from 2 previous studies with larger sample sizes. These studies were performed by Zhang et al. [27] and Jiang et al. [17].
The 3 studies are similar in terms of the patients’ demographics (including age and sex) and clinical characteristics (tumor location and number of tumor segments), but different in terms of the surgical outcomes. Table 3 summarizes the findings from these 3 studies. The removal rates in our study differ from those observed in the 2 previous studies, which revealed excellent removal rates. However, the recurrence rate is almost the same among the 3 studies. Conversely, Jiang et al. reported an excellent total removal rate of 81%, but a recurrence rate of 37.5%, which is almost twice as high as that noted in our study. This indicates that the removal rate does not contribute to the recurrence rate. Because the rate of neurological worsening is also unchanged in the 3 studies, the basic surgical strategy remains the same: if the neurological findings do not worsen, maximal removal can be performed. The 2 previous studies were single centered in nature and obtained very good surgical outcomes; however, such good outcomes are difficult to obtain in a multicenter study with different surgeons, such as ours. Progression-free survival, by definition, should be calculated from the total number of cases; however, Jiang et al. [17], and Zhang et al. [27] only considered recurrent cases. Therefore, it is not possible to directly compare our findings with the findings from their studies; such comparisons are possible if the progression-free survival in these previous studies is calculated correctly according to the aforementioned definition.
In conclusion, the removal rate does not contribute to the recurrence rate; if the neurological findings do not worsen, maximal removal can be performed (Table 3).
Genetic examination of the tumors could not be performed due to the study’s multicentered, retrospective nature. Furthermore, this rarity of this tumor limits our sample size, even for a multicenter study. Further investigations into the molecular biomarkers of recurrence or prognosis are indispensable. Despite these limitations, we believe that the present study is one of the largest case series with a relatively long-term follow-up and detailed data. It provides important information regarding the treatment strategy for spinal PAs.
The limited sample size (n=18) restricts the study’s statistical power and may compromise the reliability of its conclusions. The retrospective nature of the study could introduce potential biases, such as selection or information bias, which may affect the study’s validity. The wide age range of the included patients (3–73 years) may lead to confounding, as various age groups could present with different clinical manifestations or outcomes. The nonspecific imaging features of spinal PAs pose difficulties in establishing a definitive diagnosis, and may have resulted in misclassification of cases within the study.
Primary spinal PAs are rare, eccentric, and intermixed cystic and solid intramedullary cervical tumors. Surgical resection with prevention of neurological deterioration can be the first-line treatment, but the resection rate does not affect recurrence-free survival. The imaging features of spinal PAs are nonspecific, and a definitive diagnosis requires pathological support. Investigation of molecular biomarkers is required to elucidate the regrowth risk and prognostic factors. Future studies must investigate the significance of chemotherapy and radiotherapy in the treatment of PAs. Furthermore, the treatment of recurrent tumors should be established with the information of molecular biomarker.
NOTES
ACKNOWLEDGEMENTS
We really appreciate all the members in 58 neurosurgical center in Japan in Neurological society of Japan to collect the patients’ data in detail.
Fig. 1.
Magnetic resonance imaging (MRI) findings of 2 cases of recurrence. In case #5, preoperative MRI on T2WI (A) and Gd enhancement in (B) sagittal and (C) axial T1WI, after the biopsy (D). MRI at regrowth on T2WI (E) and T1WI with Gd enhancement (F). In 13-month follow-up term on T2WI (G) and T1WI with Gd enhancement (H). In case #13, on preoperative MRI on sagittal T1WI (I) and sagittal T2WI (J), and axial (K) and saggital (L) T1WI with Gd enhancement. MRI at tumor regrowth on T2WI (M) and saggital T1WI with Gd enhancement (N). After 122-month survival, T2WI (O) and T1WI with Gd enhancement (P). Gd, gadolinium; T2WI, T2-weighted imaging; T1WI, T1-weighted imaging.

Fig. 2.
Kaplan-Meier curve of recurrence-free survival in the GTR and non-GTR groups. There are no significant differences between the GTR and non-GTR (STR, PR, and biopsy) groups (p=0.94). GTR, gross total resection; STR, subtotal resection; PR, partial resection.
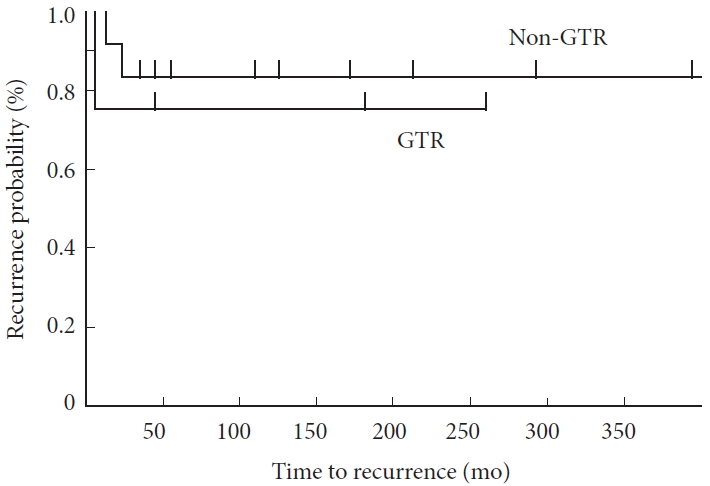
Table 1.
Demographic data of 18 patients with spinal pilocytic astrocytoma
Table 2.
Radiographic data of 18 patients with spinal pilocytic astrocytoma
Table 3.
The comparison of neurological changes and removal rates among the 2 major previous literatures
| Study | No. of cases |
Neurological changes between preoperation and postoperation |
Recurrence rate (%) |
Removal rate |
Follow-up (mo) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Improved (%) | Unchanged (%) | Deterioration (%) | Death (%) | GTR (%) | STR (%) | PR (%) | Biopsy (%) | ||||
| Zhang et al. [27] | 20 | 25 | 50 | 25 | 10 | 20 | 55 | 45 | - | - | 104 ± 56 |
| Jiang et al. [17] | 16 | 6 | 75 | 19 | 0 | 37.5 | 81 | 19 | - | - | 40.4 ± 30.4 |
| Our data | 18 | 28 | 56 | 16 | 0 | 22 | 28 | 33 | 33 | 5 | 65 ± 49 |
REFERENCES
1. Burkhard C, Di Patre PL, Schüler D, et al. A populationbased study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg 2003;98:1170-4.


2. Johnson DR, Brown PD, Galanis E, et al. Pilocytic astrocytoma survival in adults: analysis of the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. J Neurooncol 2012;108:187-93.



3. Horbinski C, Oakley GJ, Cieply K, et al. The prognostic value of Ki-67, p53, epidermal growth factor receptor, 1p36, 9p21, 10q23, and 17p13 in skull base chordomas. Arch Pathol Lab Med 2010;134:1170-6.




4. Horbinski C, Hamilton RL, Lovell C, et al. Impact of morphology, MIB-1, p53 and MGMT on outcome in pilocytic astrocytomas. Brain Pathol 2010;20:581-8.


5. Bond KM, Hughes JD, Porter AL, et al. Adult pilocytic astrocytoma: an institutional series and systematic literature review for extent of resection and recurrence. World Neurosurg 2018;110:276-83.


6. Tabash MA. Characteristics, survival and incidence rates and trends of pilocytic astrocytoma in children in the United States; SEER-based analysis. J Neurol Sci 2019;400:148-52.


7. Horger M, Ritz R, Beschorner R, et al. Spinal pilocytic astrocytoma: MR imaging findings at first presentation and following surgery. Eur J Radiol 2011;79:389-99.


8. Munshey A, Moore J, Maclean C, et al. Cranial pilocytic astrocytoma with spinal drop metastasis in an adult: case report and literature review. World Neurosurg 2017;98:883.e7-12.


9. Rashad S, Elwany A, Farhoud A. Surgery for spinal intramedullary tumors: technique, outcome and factors affecting resectability. Neurosurg Rev 2018;41:503-11.



10. Bansal S, Borkar SA, Mahapatra AK. Hydrocephalus associated with spinal intramedullary pilocytic astrocytoma. Asian J Neurosurg 2017;12:217-9.



11. Baran O, Kasimcan O, Sav A, et al. Holocord pilocytic astrocytoma in an adult: a rare case report and review of the literature. World Neurosurg 2019;126:369-75.


12. Bell E, Kanodia AK, Gunaratne B, et al. Leptomeningeal dissemination of spinal pilocytic astrocytoma: a rare entity. BMJ Case Rep 2018;2018:bcr2018226955.



13. Choi GH, Oh JK, Kim TY, et al. The clinical features and surgical outcomes of pediatric patients with primary spinal cord tumor. Childs Nerv Syst 2012;28:897-904.



14. Colnat-Coulbois S, Klein O, Braun M, et al. Management of intramedullary cystic pilocytic astrocytoma with rhenium186 intracavitary irradiation: case report. Neurosurgery 2010;66:E1023-4. discussion E4.

15. Ebner FH, Schittenhelm J, Roser F, et al. Management of holocord pilocytic astrocytomas in children and adolescents: an update. Pediatr Neurosurg 2012;48:133-40.



16. Harraher CD, Vogel H, Steinberg GK. Spinal pilocytic astrocytoma in an elderly patient. World Neurosurg 2013;79:799.E7-9.


17. Jiang Y, Lv L, Yin S, et al. Primary spinal pilocytic astrocytoma: clinical study with long-term follow-up in 16 patients and a literature review. Neurosurg Rev 2020;43:719-27.



18. Martinelli C, Gabriele F, Manai F, et al. The search for molecular markers in a gene-orphan case study of a pediatric spinal cord pilocytic astrocytoma. Cancer Genomics Proteomics 2020;17:117-30.



19. McBride D, Aljuboori Z, Hattab EM, et al. An elderly patient presenting with a primary spinal multifocal intradural extramedullary pilocytic astrocytoma: a case report and review of the literature. BMC Cancer 2018;18:806.




20. She DJ, Lu YP, Xiong J, et al. MR imaging features of spinal pilocytic astrocytoma. BMC Med Imaging 2019;19:5.




21. Bannykh SI, Mirocha J, Nuno M, et al. V600E BRAF mutation in pilocytic astrocytoma is associated with a more diffuse growth pattern but does not confer a more aggressive clinical behavior. Clin Neuropathol 2014;33:388-98.


22. Diaz-Aguilar D, ReFaey K, Clifton W, et al. Prognostic factors and survival in low grade gliomas of the spinal cord: a population-based analysis from 2006 to 2012. J Clin Neurosci 2019;61:14-21.


23. Karikari IO, Nimjee SM, Hodges TR, et al. Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery 2011;68:188-97. discussion 97.


24. Azad TD, Pendharkar AV, Pan J, et al. Surgical outcomes of pediatric spinal cord astrocytomas: systematic review and meta-analysis. J Neurosurg Pediatr 2018;22:404-10.


25. Endo T, Inoue T, Mizuno M, et al. Current trends in the surgical management of intramedullary tumors: a multicenter study of 1,033 patients by the Neurospinal Society of Japan. Neurospine 2022;19:441-52.




26. McCormick PC, Torres R, Post KD, et al. Intramedullary ependymoma of the spinal cord. J Neurosurg 1990;72:523-32.


27. Zhang L, Li T, Qiao G, et al. Clinical characteristics and longterm surgical outcomes of spinal pilocytic astrocytoma: a report of twenty cases. Acta Neurochir (Wien) 2021;163:3005-13.



28. Dauleac C, Messerer R, Obadia-Andre N, et al. Cysts associated with intramedullary ependymomas of the spinal cord: clinical, MRI and oncological features. J Neurooncol 2019;144:385-91.




- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























