|
 |
- Search
|
|
||
Abstract
Methods
A self-controlled case series analysis was conducted using the National Health Insurance Service-National Sample Cohort database in South Korea. We identified patients aged 18 years or older who had at least one prescription of limaprost and were diagnosed with at least one case of bleeding between 2003 and 2019. The incidence rate ratio (IRR) of bleeding was calculated by dividing the incidence rate in the exposed period to limaprost by that in the unexposed period and adjusted for age using conditional Poisson regression model.
Results
Among 72,860 patients with limaprost prescriptions and bleeding diagnoses, there were 184,732 events of bleeding. After adjusting for age, the IRR was 1.47 (95% confidence interval [CI], 1.43ŌĆō1.50), wherein the IRR was the highest during the 0ŌĆō7 days after limaprost initiation (IRR, 2.11; 95% CI, 2.03ŌĆō2.18). Risk of bleeding was higher when limaprost was concomitantly used with antithrombotics or other drugs for spinal stenosis treatment, and when higher daily doses of limaprost were administered.
Conclusion
Our findings suggest that the risk of bleeding increased by 1.5-fold in periods of limaprost exposure compared to unexposed periods, with particularly higher risks observed during the first week after limaprost initiation, with concomitant drugs related to bleeding, and with a higher daily dose. A careful risk-benefit assessment is warranted when initiating limaprost, especially when administered with other medications or in higher daily doses.
Limaprost, a prostaglandin E1 analog, was approved in Japan for the treatment of symptoms associated with thromboangiitis obliterans (TAO, BuergerŌĆÖs disease) and lumbar spinal stenosis (LSS) [1], and is currently available for use in Korea, Japan, and Thailand.
TAO is an occlusive disease of small- and medium-sized, distal vessels of the extremities, and causes ischemic symptoms such as ulcers, pain, and the sensation of coldness of the hands and feet. Smoking cessation is the most important treatment for TAO, but prostaglandin E1 (e.g., limaprost) improve microcirculation for TAO patients with critical ischemia [2]. LSS is a degenerative disease of the narrowing of the spinal canal results in the compression of the lumbosacral nerve roots or cauda equina, and causes leg pain, leg numbness, and/or low back pain [3-5]. Although surgical therapy is commonly needed for severe LSS, the following pharmacologic therapies are prescribed to patients with mild to moderate LSS or contraindicated surgical therapy [6,7]: nonsteroidal anti-inflammatory drugs (NSAIDs) [8], corticosteroids [9], analgesics, muscle relaxants, neuropathic drugs [10,11], and prostaglandins (e.g., limaprost) [8,11,12]. In particular, limaprost alleviates subjective symptoms of LSS by improving blood flow to the limbs and enhances the supply of nutrients to the cauda equina. Although limaprost improves symptoms associated with TAO and LSS through vasodilation, it also has the potential to cause bleeding and may act as a risk factor for bleeding during spinal surgery due to its antiplatelet action [13-16]. Additionally, patients exposed to this drug are mostly older adults, who have a higher probability of comorbidities or use comedications that elevate their risk of hemorrhage [17].
Premarket clinical trials of limaprost conducted in Japan had previously reported several hemorrhagic events of colostomy bleeding and hemoptysis [18], and similar adverse events of epistaxis, hematuria, subcutaneous hemorrhage, hemorrhagic duodenal ulcer, subarachnoid hemorrhage, and cerebral hemorrhage in postmarket surveillance (PMS) studies [19]. However, the number of participants included in these studies was very small, hindering the determination of any causal relations between limaprost exposure and the risk of bleeding. Moreover, in PMS studies, a case of epistaxis occurred in one individual who received antiplatelets together, and another case of epistaxis and subconjunctival hemorrhage was reported after the combined use of limaprost and paroxetine [20]; However, it remains unclear as to how much of the bleeding risk was increased when limaprost was used together with other drugs compared with the use of limaprost alone. Considering the lack of available literature on the potential association between limaprost and the risk of bleeding, we aimed to examine this association using a self-controlled case series (SCCS) design, which controls for time-invariant potential confounders.
We used South KoreaŌĆÖs National Health Insurance Service-National Sample Cohort (NHIS-NSC) database [21]. This database contains anonymized information on sociodemographics, diagnoses, and prescribed drugs of inpatients and outpatients for over one million individuals from 2002 to 2019. The NHIS-NSC database is also linked to the vital statistics of the Korean Statistical Office, enabling the identification of the date and cause of deaths.
The study was approved by the Institutional Review Board (IRB) of Sungkyunkwan University (SKKU 2021-11-003), and the requirement of obtaining informed consent from the study population was waived by the IRB.
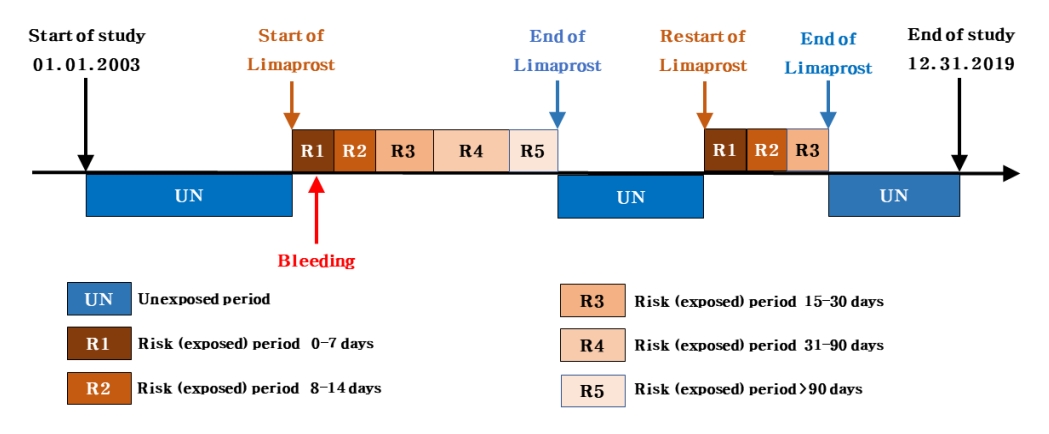
We used a SCCS design to investigate the risk of bleeding associated with limaprost use. In this design, the incidence rate of bleeding events during periods of limaprost exposure is compared with that during periods of limaprost nonexposure. Considering that individuals act as their own control (hence, are self-controlled), this design can minimize the effects of any unmeasured time-invariant variables (e.g., sex or genetic factors) (Fig. 1) [22].
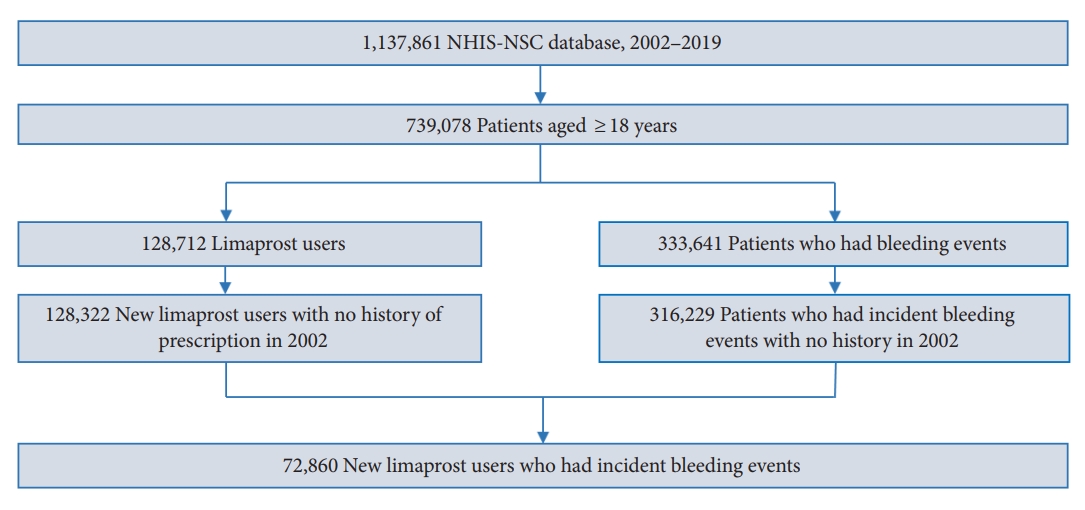
We initially identified a base cohort of adults aged 18 years or older in 2002. Subsequently, from the base cohort, we selected a study cohort of individuals who had at least one prescription of limaprost and were also diagnosed with a bleeding event at least once during the study period from January 1, 2003 to December 31, 2019 (Fig. 2). Among these patients, those prescribed limaprost or diagnosed with a hemorrhagic event in 2002 were excluded from the study cohort to restrict the subjects to new users of limaprost experiencing incident bleeding. The observation period ended on the date of death or end of the study period (December 31, 2019), whichever occurred earlier.
In South Korea, only a single formulation and dosage for limaprost exist (5-╬╝g tablet). Consecutive prescriptions of limaprost were considered single, continued prescriptions by summing up the dayŌĆÖs supply of each prescription. The end date of limaprost prescription was calculated by adding the dayŌĆÖs supply to the start date of prescription. The daily dose of limaprost was calculated as the frequency of doses multiplied by 1 dose (daily dose = 1 dose ├Ś frequency of doses); if the daily dose was missing, it was calculated using the total amount, unit price, and duration of administration (total amount=unit price├Śdaily dose├Śduration). Despite such efforts, we excluded all prescriptions of limaprost that did not include the daily dose or duration information from the study.
Risk periods, or periods of exposure to limaprost, were classified by considering its administration duration and time for maximal concentrations (Tmax). Accordingly, the risk (exposed) periods were divided into 5 categories as follows: 0ŌĆō7, 8ŌĆō14, 15ŌĆō30, 31ŌĆō90, and more than 90 days. The remaining time considered as the unexposed period.
The main outcome of interest included all types of bleeding, except traumatic or secondary bleeding, with a clear cause. Bleeding was divided into 6 subgroups according to their site of occurrence (intracranial, gastrointestinal, ocular, genitourinary, otolaryngeal, and other sites): intracranial bleeding (International Classification of Diseases, 10th revision, codes I60-I62), gastrointestinal bleeding (codes K22.6, K25.0, K25.2, K25.4, K25.6, K26.0, K26.2, K26.4, K26.6, K27.0, K27.2, K27.4, K27.6, K28.0, K28.2, K28.4, K28.6, K29.0, K62.5, K66.1, K76.2, and K92.0-K92.2), ocular bleeding (codes H11.3, H31.3, H35.6, H43.1, and H45.0), genitourinary bleeding (codes N02, N42.1, N92.0, N92.1, N92.3, N92.4, N93.8, N93.9, N95.0, and R31), otolaryngeal bleeding (codes H92.2 and R04), and bleeding at other sites (codes D69.9, I31.2, I85.0, J94.2, M25.0, and R58). Multiple bleeding events were considered separate events if they occurred at different sites or occurred at the same site more than 3 months after a preceding bleeding event.
With the SCCS design inherently controlling for unmeasured, time-invariant and between-individual confounding factors, we only considered potential time-varying confounders of age, concomitant drug use (anticoagulants, antiplatelets, thrombolytics, NSAIDs, glucocorticoids, analgesics, gabapentinoids, and muscle relaxants), and daily dose of limaprost. In particular, anticoagulants, antiplatelets, and thrombolytics are drugs with a high bleeding potential and the remaining drugs are commonly used in combination with limaprost for patients with spinal stenosis. We selected formulations of concomitant drugs as oral, injection, and patch formulations that exhibit systemic action.
We used conditional Poisson regression models to estimate unadjusted (crude) incidence rate ratios (IRRs) with a 95% confidence interval (CI), as well as IRRs that were adjusted for age (adjusted IRRs [aIRRs]), to compare the incidence rate of bleeding during the exposed period with that during the unexposed period. We used SAS Enterprise Guide 8.3 in Korean (SAS Institute Inc., Cary, NC, USA) for data management and statistical analysis.
Various subgroup analyses were conducted for sex, presence of spinal stenosis, bleeding sites, concomitant drugs, and the daily dose of limaprost. For the analysis of concomitant drugs, we compared the incidence rates of bleeding between 3 different periods: periods without exposure to limaprost, periods with exposure to limaprost alone, and periods with exposure to both limaprost and concomitant drugs of interest (e.g., antithrombotics). The recommended daily doses of limaprost are 15 ╬╝g for LSS treatment, and 30 ╬╝g for TAO treatment [23]. Therefore, the analysis of the daily dose of limaprost was classified into low dose (daily dose Ōēż 15 ╬╝g), medium dose (15 ╬╝g < daily dose Ōēż 30 ╬╝g), and high dose (daily dose > 30 ╬╝g).
We also conducted multiple sensitivity analyses to determine the robustness of our main findings. First, to exclude the potential effect of the first bleeding event on subsequent bleeding events, we considered only the first bleeding event as the outcome [24]. Additionally, we altered the outcome definition by considering bleeding events that occurred at the same site more than one year after a preceding bleeding event as a new event. Second, we excluded all individuals who died during the study period (including those who died within 60 days of the first bleeding event), as the observation period for these individuals may be shortened, which could then lead to bias by violating an assumption of the SCCS design [24]. Third, we excluded patients with cancer records because cancer may affect the hemostatic system [25]. Fourth, in order to account for the patientsŌĆÖ compliance to their prescribed medication, we introduced 3 consecutive washout periods (1ŌĆō7, 8ŌĆō14, and 15ŌĆō30 days) to the end of the last risk period. We also introduced 3 consecutive pre-exposure periods (1ŌĆō7, 8ŌĆō14, and 15ŌĆō30 days) preceding the risk period to eliminate any bias arising from event-dependent exposure [24]. If a washout period and a pre-exposure period overlapped, the latter was included as part of washout period. Fifth, we analyzed a potentially vague time relationship by separating the bleeding events that occurred on the same date as that of prescription for limaprost. Last, we set a 7-day grace period between prescriptions to ensure medication compliance.
Among the 739,078 adults aged 18 years or older, 72,860 patients were prescribed limaprost and had at least one record of bleeding (Fig. 2). The number of deaths during the study period was 8,322 (11.42%). There were 70,791 (97.16%), 65,040 (89.27%), and 19,130 (26.26%) patients with spinal canal stenosis, dyslipidemia, and cancer, respectively. The most commonly used drug with limaprost was NSAIDs (92.83%), followed by analgesics (69.90%) and muscle relaxants (68.02%). The median age at the time of first limaprost exposure was 64 years, which was older than that at the time of first bleeding (61 years). During a mean follow-up duration of 16.53 years, the median total duration of limaprost exposure was 34 days. In total, 184,732 events of bleeding occurred during the study period, of which, gastrointestinal bleeding was the most common, with 63,542 events of bleeding (Table 1).
The unadjusted IRR for total bleeding during the period of limaprost exposure, relative to the unexposed period, was 1.69 (95% CI, 1.65ŌĆō1.73). After adjusting for age, the adjusted IRR (aIRR) was slightly attenuated to 1.47 (95% CI, 1.43ŌĆō1.50). In the analysis that divided the risk (exposed) period based on the administration duration of limaprost, the aIRR for total bleeding was the highest during the 0- to 7-day period (aIRR, 2.11; 95% CI, 2.03ŌĆō2.18) and showed a fluctuating risk profile throughout the 90-day period (Table 2).
Results of subgroup analyses showed that the aIRR for total bleeding did not differ by sex (male: aIRR, 1.52; 95% CI, 1.47ŌĆō1.58; female: aIRR, 1.42; 95% CI, 1.37ŌĆō1.46). However, the aIRR was slightly lower in patients with spinal stenosis compared to patients without spinal stenosis (patients with spinal stenosis: aIRR, 1.46; 95% CI, 1.43ŌĆō1.50; patients without spinal stenosis: aIRR, 1.91; 95% CI, 1.46ŌĆō2.52). Considering the risk of bleeding by site, the aIRR values were the highest for intracranial bleeding (aIRR, 1.78; 95% CI, 1.62ŌĆō1.95) and the lowest for ocular bleeding (aIRR, 1.14; 95% CI, 1.08ŌĆō1.20) (Table 2). When antithrombotic agents (anticoagulants, antiplatelets, and thrombolytics) were used in combination with limaprost, the bleeding risk was higher than that of limaprost use alone (anticoagulants: aIRR, 3.22 vs. 1.41; antiplatelets: aIRR, 1.59 vs. 1.41; thrombolytics: aIRR, 77.92 vs. 1.47). When limaprost was used together with other drugs for spinal stenosis treatment (NSAIDs, corticosteroids, analgesics, gabapentinoids, and muscle relaxants), the bleeding risk was also higher than that of limaprost use separately (NSAIDs: aIRR, 1.59 vs. 1.22; corticosteroids: aIRR, 2.99 vs. 1.33; analgesics: aIRR, 1.79 vs. 1.23; gabapentinoids: aIRR, 1.57 vs. 1.44; muscle relaxants: aIRR, 1.77 vs. 1.33). In the analysis that explored a dose-response relation, the risk of bleeding was the lowest at a low dose (Ōēż 15 ╬╝g/day) of limaprost (aIRR, 1.46; 95% CI, 1.43ŌĆō1.50), and slightly increased at a medium dose (aIRR, 1.64; 95% CI, 1.44ŌĆō1.87), whereas the risk significantly increased at a high dose (> 30 ╬╝g/day) (aIRR, 46.41; 95% CI, 17.42ŌĆō123.70) (Table 3).
Results of various sensitivity analyses were generally similar to those of the main analysis. Notably, when considering only the first bleeding event as the outcome, the age-adjusted IRR was slightly lower than that of the main analysis (aIRR, 1.20; 95% CI, 1.15ŌĆō1.26). When separately analyzing bleeding events that occurred on the same date as that of limaprost administration, the age-adjusted IRR increased considerably to 7.36 (95% CI, 6.99ŌĆō7.74) (Table 4).
In this nationwide study that used a SCCS design, the risk of bleeding was approximately 1.5-fold higher in periods of exposure to limaprost compared with unexposed periods, with particularly higher risks observed in the first week of treatment initiation. Subgroup analyses showed that the risk of bleeding associated with limaprost initiation increased with the use of concomitant drugs, and also exhibited a clear dose-response association. Therefore, we inferred that the higher risk of bleeding during limaprost exposure periods is likely attributed to its antiplatelet function. Furthermore, the highest risk of bleeding during the first week, especially on the day of administration, can be attributed to the fact that time for maximal concentrations (Tmax) is brief at 0.5 hour [13] and because NSAIDs or steroids are also commonly used in combination at the beginning of limaprost administration.
To date, studies on limaprost have largely focused on its therapeutic effects and less on its potential risks, especially bleeding. Studies on the risk of adverse events with limaprost use were conducted through premarket clinical trials and PMS studies in Japan, and several cases of bleeding were reported. Additionally, epistaxis or hemorrhagic duodenal ulcer occurred in individuals who concomitantly used antiplatelets or NSAIDs [19]. However, the number of participants included in these studies were not sufficient to deduce generalizable results, and only the overall occurrence of side effects was confirmed. To the best of our knowledge, however, this population-based study is the first and largest to have investigated the possible association between the initiation of limaprost and the risk of bleeding in a nationwide setting that controlled for various time-invariant confounders.
In a post hoc analysis on the use of antiplatelet therapy and the early time course of major bleeding, Hilkens et al. [26] found that the incidence of major bleeding was highest in dual antiplatelet therapy and during the first 30 days of administration. This result is consistent with our findings, wherein the risk of bleeding was higher with concomitant drug use (including antiplatelets) and during the 0- to 30-day period (aIRR, 1.57; 95% CI, 1.52ŌĆō1.61) compared with the 31- to 90-day period (aIRR, 1.20; 95% CI, 1.14ŌĆō1.27). However, in our study, the first 30 days of limaprost use were further subdivided into 0ŌĆō7, 8ŌĆō14, and 15ŌĆō30 days considering its rapid action (Tmax was 0.5 hour and the inhibition of platelet aggregation occurred after 5 days of continuous administration [27]); the risk of bleeding was the highest in the first 0ŌĆō7 days after limaprost initiation (aIRR, 2.11; 95% CI, 2.03ŌĆō2.18).
In the SCCS study on respiratory tract infection and risk of bleeding in anticoagulant users, Ahmed et al. [28] observed a greater than twofold increase in the risk of major bleeding and clinically relevant nonmajor bleeding in the 0ŌĆō14 days after an untreated respiratory infection. This result is consistent with our findings, wherein the risk of bleeding in all sites was highest during the 0- to 7-day period (intracranial bleeding: aIRR, 3.56; 95% CI, 3.12ŌĆō4.08; gastrointestinal bleeding: aIRR, 2.40; 95% CI, 2.27ŌĆō2.55; ocular bleeding: aIRR, 1.28; 95% CI, 1.16ŌĆō1.40; genitourinary bleeding: aIRR, 2.40; 95% CI, 2.23ŌĆō2.58; otolaryngeal bleeding: aIRR, 1.49; 95% CI, 1.31ŌĆō1.68; bleeding at other sites: aIRR, 2.98; 95% CI, 2.57ŌĆō3.46). However, Ahmed et al. divided outcomes into major bleeding and clinically relevant nonmajor bleeding events, based on the severity of bleeding. In contrast, our study included all types of bleeding in the main analysis and categorized these events into 6 groups (intracranial, gastrointestinal, ocular, genitourinary, otolaryngeal bleeding, and bleeding at other sites) in the subgroup analysis based on the site of bleeding. This was done because the causes and treatments vary depending on the specific bleeding sites.
Limaprost, a prostaglandin E1 analog, inhibits platelet adhesiveness and aggregation when orally administered in patients with thrombotic diseases [29]. In guinea-pigs (in vitro), limaprost inhibits platelet aggregation induced by various aggregating agents and dissociates adenosine diphosphate-induced platelet aggregation [30]. Limaprost also increases the threshold voltage of thrombosis formation in the mesenteric artery of guinea-pigs with thrombosis formation induced by electric stimulation [31].
The main strength of this study is the use of a SCCS design to minimize any bias occurring from time-invariant confounders. To further examine the effects of time-varying confounders, the effect estimates were adjusted for age, and various clinically meaningful subgroup analyses (e.g., concomitant drugs and daily dose) were conducted. Finally, a range of sensitivity analyses were conducted to assess the robustness of the main results. Nevertheless, this study has several limitations, that should be considered when interpreting our results. First, when considering only the first bleeding event as the outcome, the age-adjusted IRR was 1.20 (95% CI, 1.15ŌĆō1.26), which was lower than that of the main analysis (IRR, 1.47; 95% CI, 1.43ŌĆō1.50). This underestimated result could be related to the occurrence of the first bleeding event before limaprost administration because the number of products and prescriptions of limaprost were lower in the early study period (2003ŌĆō2010) compared with the later study period (2011ŌĆō2019) in Korea [32]. However, the additional sensitivity analysis that considered bleeding events occurring more than 1 year after a preceding bleeding event at the same site as a new event revealed an age-adjusted IRR of 1.44 (95% CI, 1.40ŌĆō1.48), which was similar to that of the main analysis. Second, we did not consider the use of gastroprotective drugs, which could have subsequently contributed to the prevention of gastrointestinal bleeding [33]. Hence, further studies are required to compare the effect of concomitant use of gastroprotective drugs with limaprost on bleeding risks. Third, we did not consider the severity of bleeding owing to the use of claims data. Fourth, patients may not have taken the limaprost prescribed. However, any bias arising from this is likely minimal, as a sensitivity analysis that applied a 7-day grace period between prescriptions found that our results (aIRR, 1.45; 95% CI, 1.41ŌĆō1.48) were consistent with those of the main analysis. Fifth, some NSAIDs, analgesics, and muscle relaxants may have been used with limaprost as over-the-counter medications; however, it is common local practice to coprescribe these medications with limaprost. Sixth, selective serotonin reuptake inhibitors (SSRIs), which have a high bleeding tendency among antidepressants, were not considered as concomitant drugs because SSRIs are unlikely to be used in combination with limaprost for the treatment of LSS. Last, despite the use of a SCCS design, residual effects from unmeasured or unaccounted confounders may be present.
In this study, the risk of bleeding was approximately 1.5-fold higher in limaprost exposure periods compared with unexposed periods, with a particularly higher risk being observed in the first week of limaprost treatment. The results of the subgroup analysis showed that the risk of bleeding increased when limaprost was used together with other drugs or when taken at higher daily doses. Considering that limaprost prescriptions are continuously increasing in Korea, our results provide important realworld evidence that could help clinicians in their decision-making by recommending minimal co-prescriptions with limaprost and its appropriate dose prescriptions.
NOTES
Fig.┬Ā2.
Selection of the study participants. NHIS-NSC, National Health Insurance Service-National Sample Cohort.
Table┬Ā1.
Characteristics of patients with bleeding events
| Characteristic | Value |
|---|---|
| No. of patients | 72,860 |
| Male sex | 29,637 (40.68) |
| No. of deaths during the study period | 8,322 (11.42) |
| No. of patients with comorbidityŌĆĀ | |
| ŌĆāSpinal canal stenosis | 70,791 (97.16) |
| ŌĆāHypertension | 54,144 (74.31) |
| ŌĆāDiabetes mellitus | 51,181 (70.25) |
| ŌĆāDyslipidemia | 65,040 (89.27) |
| ŌĆāCerebrovascular disease | 31,149 (42.75) |
| ŌĆāPeripheral vascular disease | 61,067 (83.81) |
| ŌĆāCancer | 19,130 (26.26) |
| No. of patients with comedicationŌĆĪ | |
| ŌĆāAnticoagulants | 5,121 (7.03) |
| ŌĆāAntiplatelets | 24,407 (33.50) |
| ŌĆāThrombolytics | 69 (0.09) |
| ŌĆāNSAIDs | 67,636 (92.83) |
| ŌĆāSteroids | 40,556 (55.66) |
| ŌĆāAnalgesics | 50,931 (69.90) |
| ŌĆāGabapentinoids | 19,227 (26.39) |
| ŌĆāMuscle relaxants | 49,557 (68.02) |
| Age (yr) | |
| ŌĆāStudy entry date | 54 (45ŌĆō63) |
| ŌĆāFirst exposure | 64 (55ŌĆō72) |
| ŌĆāFirst outcome | 61 (52ŌĆō70) |
| Duration of total exposure (day) | 34 (12ŌĆō117) |
| Duration of follow-up (yr) | 16.53 ┬▒ 1.63 |
| No. of bleeding events | 184,732 |
| ŌĆāIntracranial bleeding | 7,913 (4.28) |
| ŌĆāGastrointestinal bleeding | 63,542 (34.40) |
| ŌĆāOcular bleeding | 46,144 (24.98) |
| ŌĆāGenitourinary bleeding | 37,283 (20.18) |
| ŌĆāOtolaryngeal bleeding | 22,609 (12.24) |
| ŌĆāOther sites bleeding | 7,241 (3.92) |
Table┬Ā2.
Risk of bleeding in limaprost users
| Variable | No. of events | Person-years |
IRR (95% CI) |
|
|---|---|---|---|---|
| Unadjusted | Age-adjusted | |||
| Unexposed | 177,436 | 1,176,041 | 1 (reference) | 1 (reference) |
| Exposed (day) | 7,296 | 28,557.81 | 1.69 (1.65ŌĆō1.73) | 1.47 (1.43ŌĆō1.50) |
| ŌĆā0ŌĆō7 | 2,933 | 8,172.22 | 2.38 (2.29ŌĆō2.47) | 2.11 (2.03ŌĆō2.18) |
| ŌĆā8ŌĆō14 | 877 | 4,367.46 | 1.33 (1.25ŌĆō1.42) | 1.17 (1.09ŌĆō1.25) |
| ŌĆā15ŌĆō30 | 1,212 | 6,113.32 | 1.31 (1.24ŌĆō1.39) | 1.14 (1.08ŌĆō1.21) |
| ŌĆā31ŌĆō90 | 1,414 | 6,621.73 | 1.42 (1.34ŌĆō1.49) | 1.20 (1.14ŌĆō1.27) |
| ŌĆāŌēź 91 | 860 | 3,283.08 | 1.74 (1.62ŌĆō1.86) | 1.44 (1.35ŌĆō1.54) |
| Male | ||||
| ŌĆāUnexposed | 74,452 | 473,607.80 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 3,265 | 11,353.10 | 1.83 (1.77ŌĆō1.89) | 1.52 (1.47ŌĆō1.58) |
| Female | ||||
| ŌĆāUnexposed | 102,984 | 702,433.30 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 4,031 | 17,204.71 | 1.60 (1.55ŌĆō1.65) | 1.42 (1.37ŌĆō1.46) |
| Spinal stenosisŌĆĀ (yes) | ||||
| ŌĆāUnexposed | 172,887 | 1,142,653 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 7,244 | 28,381.58 | 1.69 (1.65ŌĆō1.73) | 1.46 (1.43ŌĆō1.50) |
| Spinal stenosisŌĆĀ (no) | ||||
| ŌĆāUnexposed | 4,549 | 33,387.93 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 52 | 176.23 | 2.17 (1.65ŌĆō2.85) | 1.91 (1.46ŌĆō2.52) |
| Intracranial | ||||
| ŌĆāUnexposed | 7,449 | 53,275.97 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 464 | 1,807.30 | 1.84 (1.67ŌĆō2.02) | 1.78 (1.62ŌĆō1.95) |
| Gastrointestinal | ||||
| ŌĆāUnexposed | 61,046 | 578,273.80 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 2,496 | 14,612.74 | 1.62 (1.55ŌĆō1.68) | 1.49 (1.43ŌĆō1.55) |
| Ocular | ||||
| ŌĆāUnexposed | 44,731 | 421,574.30 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 1,413 | 10,438.61 | 1.28 (1.21ŌĆō1.35) | 1.14 (1.08ŌĆō1.20) |
| Genitourinary | ||||
| ŌĆāUnexposed | 35,412 | 354,291.60 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 1,871 | 8,948.13 | 2.09 (2.00ŌĆō2.19) | 1.68 (1.60ŌĆō1.76) |
| Otolaryngeal | ||||
| ŌĆāUnexposed | 21,915 | 237,396 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 694 | 5,982.02 | 1.26 (1.17ŌĆō1.36) | 1.15 (1.07ŌĆō1.25) |
| Other sites | ||||
| ŌĆāUnexposed | 6,883 | 95,682.21 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 358 | 2,585.39 | 1.92 (1.73ŌĆō2.14) | 1.73 (1.56ŌĆō1.93) |
Table┬Ā3.
Subgroup analyses for the risk of bleeding based on concomitant drug use and daily doses
Table┬Ā4.
Sensitivity analyses for the risk of bleeding
| Variable | No. of events | Person-years |
IRR (95% CI) |
|
|---|---|---|---|---|
| Unadjusted | Age-adjusted | |||
| Defining outcomes as the first bleeding only | ||||
| ŌĆāUnexposed | 70,800 | 1,176,041 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 2,060 | 28,557.81 | 1.20 (1.15ŌĆō1.25) | 1.20 (1.15ŌĆō1.26) |
| Considering outcomes that occurred in different sites or in the same site after Ōēź 1 yr | ||||
| ŌĆāUnexposed | 140,178 | 1,176,041 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 5,511 | 28,557.81 | 1.62 (1.58ŌĆō1.66) | 1.44 (1.40ŌĆō1.48) |
| Excluding patients who died during the study period | ||||
| ŌĆāUnexposed | 158,084 | 1,071,641 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 6,137 | 25,039.70 | 1.66 (1.62ŌĆō1.70) | 1.43 (1.40ŌĆō1.47) |
| Excluding patients who died Ōēż 60 days after the first bleeding during the study period | ||||
| ŌĆāUnexposed | 176,909 | 1,169,856 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 7,243 | 28,349.03 | 1.69 (1.65ŌĆō1.73) | 1.46 (1.43ŌĆō1.50) |
| Excluding patients with cancer records | ||||
| ŌĆāUnexposed | 124,483 | 876,385.20 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 4,753 | 19,916.38 | 1.68 (1.63ŌĆō1.73) | 1.46 (1.42ŌĆō1.51) |
| Including 30 days of washout periods | ||||
| ŌĆāBaselineŌĆĀ | 172,720 | 1,154,677 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 7,296 | 28,557.81 | 1.71 (1.67ŌĆō1.75) | 1.48 (1.44ŌĆō1.51) |
| ŌĆāWash outŌĆĪ | 4,716 | 21,363.96 | 1.48 (1.43ŌĆō1.52) | 1.32 (1.28ŌĆō1.36) |
| Including 30 days of pre-exposure periods and 30 days of washout periods | ||||
| ŌĆāBaseline┬¦ | 169,783 | 1,139,846 | 1 (reference) | 1 (reference) |
| ŌĆāPre-exposedll | 2,937 | 14,831.05 | 1.33 (1.28ŌĆō1.38) | 1.21 (1.16ŌĆō1.25) |
| ŌĆāExposed | 7,296 | 28,557.81 | 1.72 (1.68ŌĆō1.76) | 1.48 (1.45ŌĆō1.52) |
| ŌĆāWash out | 4,716 | 21,363.96 | 1.48 (1.43ŌĆō1.53) | 1.33 (1.29ŌĆō1.37) |
| Including 7 days of grace periods | ||||
| ŌĆāUnexposed | 177,296 | 1,175,065 | 1 (reference) | 1 (reference) |
| ŌĆāExposed | 7,436 | 29,533.71 | 1.67 (1.63ŌĆō1.71) | 1.45 (1.41ŌĆō1.48) |
| Separating the exposed period into 0 day and Ōēź 1 day | ||||
| ŌĆāUnexposed | 177,436 | 1,176,041 | 1 (reference) | 1 (reference) |
| ŌĆāExposed (0 day┬Č) | 1,492 | 1,189.90 | 8.31 (7.90ŌĆō8.75) | 7.36 (6.99ŌĆō7.74) |
| ŌĆāExposed (Ōēź 1 day) | 5,804 | 27,367.91 | 1.41 (1.37ŌĆō1.44) | 1.22 (1.18ŌĆō1.15) |
REFERENCES
1. Ono Pharmaceutical Co.. Additional indication approved for limaprost, oral prostaglandin E1 derivatives [Internet]. Osaka (Japan), Ono Pharmaceutical Co., Ltd.. 2001;[2023 Mar 8]. Available from: https://www.ono-pharma.com/sites/default/files/en/news/press/enews20010423.pdf.
2. Szuba A, Cooke JP. Thromboangiitis obliterans. An update on BuergerŌĆÖs disease. West J Med 1998;168:255-60.


5. Wei FL, Liu Y, Zhou CP, et al. Management for lumbar spinal stenosis: protocol for a network meta-analysis and systematic review. J Orthop Surg (Hong Kong) 2020;28:2309499020975212.



6. Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: conservative or surgical management?: a prospective 10-year study. Spine (Phila Pa 1976) 2000;25:1424-35. discussion 35-6.

7. Ammendolia C, Stuber KJ, Rok E, et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev 2013;(8):CD010712.

8. Takahashi J, Kobayashi H, Wakabayashi S, et al. The effect of a prostaglandin E-1 derivative on the symptoms and quality of life of patients with lumbar spinal stenosis. J Orthop Sci 2013;18:208-15.


9. Do KH, Kim TH, Chang MC. Effects of interlaminar epidural steroid injection in patients with moderate to severe lumbar central spinal stenosis: a prospective study. Ann Palliat Med 2020;9:163-8.


10. Yaksi A, Ozg├Čnenel L, Ozg├Čnenel B. The efficiency of gabapentin therapy in patients with lumbar spinal stenosis. Spine (Phila Pa 1976) 2007;32:939-42.


11. Kim HJ, Kim JH, Park YS, et al. Comparative study of the efficacy of limaprost and pregabalin as single agents and in combination for the treatment of lumbar spinal stenosis: a prospective, double-blind, randomized controlled non-inferiority trial. Spine J 2016;16:756-63.


12. Matsudaira K, Seichi A, Kunogi J, et al. The efficacy of prostaglandin E1 derivative in patients with lumbar spinal stenosis. Spine (Phila Pa 1976) 2009;34:115-20.


13. Park YS, Park JH, Kim SH, et al. Pharmacokinetic characteristics of a vasodilatory and antiplatelet agent, limaprost alfadex, in the healthy Korean volunteers. Clin Appl Thromb Hemost 2010;16:326-33.



14. Le Huec JC, AlEissa S, Bowey AJ, et al. Hemostats in spine surgery: literature review and expert panel recommendations. Neurospine 2022;19:1-12.




15. Ahlquist S, Park HY, Kelley B, et al. Venous thromboembolism chemoprophylaxis within 24 hours of surgery for spinal cord injury: is it safe and effective? Neurospine 2020;17:407-16.




16. Wang H, Yu H, Zhang N, et al. Incidence, risk factors, and management of postoperative hematoma following anterior cervical decompression and fusion for degenerative cervical diseases. Neurospine 2023;20:525-35.




17. Beyth RJ, Landefeld CS. Anticoagulants in older patients. A safety perspective. Drugs Aging 1995;6:45-54.

18. Uratsuji M, Iguchi T, Kataoka O, et al. The optimal dose for OP-1206 ŌłÖ ╬▒-CD on lumbar spinal canal stenosis: multi-center comparative double-blind clinical study. Rinsho Iyaku 1996;12:489-509. (Japanese).
19. Ono Pharmaceutical Co., Ltd. Postmarketing surveillance [Internet]. Osaka (Japan), Ono Pharmaceutical Co., Ltd.. 2009;[2023 Apr 29]. Available from: https://www.pmda.go.jp/drugs_reexam/2010/P201000115/40009300_21700AMZ00067_A100_1.pdf.
20. Sugiyama N, Sasayama D, Amano N. Massive epistaxis and subconjunctival hemorrhage due to combination of paroxetine and limaprost alfadex: a case report. Prim Care Companion J Clin Psychiatry 2007;9:240-1.


21. Lee SW, Park Y, Kim S, et al. Comorbidities of nontuberculous mycobacteria infection in Korean adults: results from the National Health Insurance Service-National Sample Cohort (NHIS-NSC) database. BMC Pulm Med 2022;22:283.




22. Hallas J, Potteg├źrd A. Use of self-controlled designs in pharmacoepidemiology. J Intern Med 2014;275:581-9.



23. Ono Pharmaceutical Co., Ltd. Opalmon Tablets 5╬╝g [Internet]. Osaka (Japan), Ono Pharmaceutical Co., Ltd.. 2010;[2023 Mar 25]. Available from: https://s3-us-west-2.amazonaws.com/drugbank/fda_labels/DB09211.PDF?1498770268.
24. Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 2016;354:i4515.


25. Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013;11:223-33.



26. Hilkens NA, Algra A, Kappelle LJ, et al. Early time course of major bleeding on antiplatelet therapy after TIA or ischemic stroke. Neurology 2018;90:e683-9.



27. Yamamoto T, Hiromoto J. Phase I clinical trial of 17(S)-methylomega-homo-trans-delta2-prostaglandinE1 ŌłÖ ╬▒-cyclodextrin (OP-1206 ŌłÖ ╬▒-CD): part 2. 5-day repeated dose study. Yakuri To Chiryo 1981;9:1935-45. (Japanese).
28. Ahmed H, Whitaker H, Farewell D, et al. Respiratory tract infection and risk of bleeding in oral anticoagulant users: selfcontrolled case series. BMJ 2021;375:e068037.



29. Maeda Y, Kanayama S, Okajima Y, et al. Effect of PGE1 analogue (ONO-1206) on the platelet functions. Blood Vessel 1982;13:142-5. (Japanese).
30. Tsuboi T, Fujitani B, Maeda J, et al. Effect of OP 1206, a prostaglandin E1 derivative, on guinea-pig platelet functions. Thromb Res 1980;20:573-80.


31. Fujitani B, Watanabe M, Kuwashima J, et al. Effect of a prostaglandin E1 derivative (OP-1206) and acetylsalicylic acid on electrically induced thrombosis in guinea-pig mesenteric artery and its modification by an inhibitor of prostaglandin I2 synthetase, tranylcypromine. Jpn J Pharmacol 1986;40:31-5.


32. Ministry of Food and Drug Safety. Search for information such as medicines [Internet]. Cheongju (Korea), Ministry of Food and Drug Safety. 2023;[2023 Mar 25]. Available from: https://nedrug.mfds.go.kr/searchDrug?sort=ITEM_PERMIT_DATE&sortOrder=true&searchYn=&ExcelRowdata=&page=1&searchDivision=detail&itemName=%EB%A6%AC%EB%A7%88%ED%94%84%EB%A1%9C%EC%8A%A4%ED%8A%B8&itemEngName=&entpName=&entpEngName=&ingrName1=&ingrName2=&ingrName3=&ingrEngName=&itemSeq=&stdrCodeName=&atcCodeName=&indutyClassCode=&sClassNo=&narcoticKindCode=&cancelCode=&etcOtcCode=&makeMaterialGb=&searchConEe=AND&eeDocData=&searchConUd=AND&udDocData=&searchConNb=AND&nbDocData=&startPermitDate=&endPermitDate.

- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2