 |
 |
- Search
| Neurospine > Volume 21(1); 2024 > Article |
|
|
Abstract
Objective
The cervical spine presents challenges in treating metastatic cervical spinal tumors (MCSTs). Although the efficacy of cervical pedicle screw placement (CPS) has been well established, its use in combination with 5.5-mm rods for MCST has not been reported. This study aimed to evaluate the efficacy of CPS combined with 5.5-mm rods in treating MCST and compare it with that of CPS combined with traditional 3.5-mm rods.
Methods
This retrospective study analyzed 58 patients with MCST who underwent posterior cervical spinal fusion surgery by a single surgeon between March 2012 and December 2022. Data included demographics, surgical details, imaging results, numerical rating scale score for neck pain, Eastern Cooperative Oncology Group performance status, and Spine Oncology Study Group Outcomes Questionnaire responses.
The spine is the most common site of cancer metastasis in the skeletal system, where metastasis spreads most commonly after the lungs and liver [1-3]. Spinal metastasis can lead to spinal instability resulting from osteolysis of the vertebrae and a loss of spinal integrity. Spinal instability can result in severe pain, neurological deficits from spinal cord compression, and spinal kyphotic deformity due to vertebral column destruction. The Spine Oncology Study Group proposed using the spinal instability neoplastic score (SINS) to evaluate spinal instability in patients with spinal metastasis [4]. The elements of the SINS include location, pain characteristics, bone lesion characteristics, radiographic spinal alignment, vertebral body collapse, and posterolateral involvement of the spinal elements. When considering the site of spinal metastasis, although it occurs in approximately 40% of patients with cancer, the cervical spine is less commonly affected, accounting for 2%–10% of all cases. However, neurological sequelae of cervical spinal metastasis can be serious even after treatment [5-9].
The cervicothoracic junction (CTJ), where approximately 10% of all spinal metastases occur, is the most challenging anatomical transitional area for treating spinal pathologies [8,10-13]. Although controversial, the consensus of surgical treatment has consistently improved biomechanical stability [12]. Given that instrumentation should cross the CTJ to access lower cervical and upper thoracic spinal metastases, considering the different biomechanical characteristics of this transitional zone is important.
Previous studies have demonstrated the efficacy and safety of cervical pedicle screw placement (CPS) in treating various spinal diseases [12-14]. We have also previously shown the accuracy and safety of CPS in treating several biomechanically challenging spinal pathologies [15-22]. This study aimed to highlight the efficacy of CPS when combined with 5.5-mm single-diameter rods and compare it with that of CPS with traditional 3.5-mm diameter rods in treating metastatic cervical spinal tumors (MCSTs) extending from the C2 vertebra to the CTJ.
The study was conducted according to the Helsinki Declaration (https://www.wma.net/policies-post/wma-declaration-ofhelsinki-ethical-principles-for-medical-research-involving-human-subjects/) and approved by the Institutional Review Board (IRB) of Asan Medical Center, and the IRB waived the requirement of obtaining informed consent for this study (AMC IRB 2023-0628).
From March 2012 to December 2022, 58 consecutive patients with MCST (37 males and 21 females), with a mean age of 60.1 years (range, 24–80 years), underwent posterior cervical spinal fusion surgery by a single surgeon for MCST.
We performed palliative spinal reconstruction using CPS for MSCT if the life expectancy was > 3 months, followed by adjuvant radiotherapy. Patients with MCSTs compressing the spinal cord from the C2 to T2 vertebrae were included in this study. All surgeries, including decompression and stabilization using CPS, were performed using the posterior approach. Anterior column support using mesh cages filled with allograft bone chips was performed in 12 patients via the anterior approach. In 3 cases of revision surgery, primary surgery was performed at a different hospital, after which tumor recurrence and instrumentation failure developed.
Two rods were used in this study: a 5.5-mm single-diameter rod combined with the Legacy pedicle screw system (Medtronic Sofamor Danek, Memphis, TN, USA), with diameters ranging from 4.0 to 6.5 mm, was used after March 2018, and a 3.5 mm single-diameter rod combined with the Vertex pedicle screw system (Medtronic Sofamor Danek), with diameters ranging from 3.5 to 4.0 mm, was used before March 2018. Although a single-diameter 5.5-mm rod and Legacy system were used to cross the CTJ in the 5.5-mm rod group, hybrid rods tapered from 3.5 to 5.5 mm and dual Vertex and Legacy systems were used to cross the CTJ in the 3.5-mm rod group before March 2018. Clinical and radiological outcomes were compared between the 2 groups.
The patients were placed in the prone position to allow for a maximally horizontal lamina plane parallel to the ground. Motor-evoked potential was monitored throughout the procedure, except under emergency conditions. The entry point of the screw was determined from the sagittal and axial computed tomography (CT) scan images: defined as the notch level in the sagittal plane and medial to the lateral border of the superior articular process by one-quarter of its width in the axial plane (or by one-half of its width at C7) [15]. A small pilot hole was created at the predetermined entry point using a 1.8-mm diameter match head burr. A small, curved pedicle probe (2.5-mm diameter) was slowly inserted vertically into the global lamina plane with a medial trajectory through the cortical hole, and the tip was placed at the thick medial cortical pedicle wall. After identifying the cancellous channel, applying a medially directed force with the probe resulted in an insertion depth of approximately 30 mm. Ball tip probe palpation was performed after forming a track with the curved probe. Next, a straight pedicle probe, tap, and screw were inserted. After tapping with a 3.5-mm diameter tap, a screw was inserted. Screw diameters ranging from 3.5 to 6.5 mm were selected based upon preoperative CT measurements from the axial images. The aforementioned procedures were performed freehand or under navigation guidance if the equipment was available. A detailed technical description of CPS has been described previously [15,16,19,21,23]. After screw insertion, the laminae were removed en bloc, and a ventral osteolytic portion of the tumor was removed using a curet and pituitary forceps through the posterolateral windows. When a large defect after the removal of the ventral osteolytic portion of the tumor created an unstable construct or when posterolateral tumor removal appeared insufficient, an additional anterior approach and mesh cage insertion were followed. However, the posterior-only approach was used in approximately 80% of cases (46 among 58 patients across both groups). Rods of varying diameters were installed after screw insertion and decompression, and acceptable instrumentation positions and alignments were identified through intraoperative x-rays and CT if available [23].
After decompression, allograft bone chips were used for posterolateral fusion.
The patients were followed up 1, 3, 6, and 12 months after surgery, and comparisons were made between the two groups. The Numeric Rating Scale (NRS) for neck pain, Eastern Cooperative Oncology Group (ECOG) performance status, and Spine Oncology Study Group Outcomes Questionnaire (SOSGOQ) responses were also compared. Preoperative SINS was obtained from all patients [4].
Radiological follow-up was performed at the same time intervals. Segmental Cobb angle was measured by x-ray at preoperative, immediate postoperative, and last follow-up times. Changes in the segmental Cobb angle (last follow-up − immediate postoperative) were calculated and determined as reduction loss. If the reduction loss was > 10°, it was classified as a collapse occurrence (Figs. 1-3) [24].
All statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Descriptive statistics are presented as frequencies, percentages, ranges, means, and standard deviations and were used to describe the background data. We conducted a normality test using the Shapiro-Wilk test on the variables. If a variable did not follow a normal distribution, we employed nonparametric tests. An independent t-test or the Mann-Whitney U-test was used for continuous data, and the chi-square test or Fisher exact test was performed for categorical data to compare the 2 groups (5.5-mm rod group vs. 3.5-mm rod group). A paired t-test (or Wilcoxon signed-rank test) was used to compare clinical outcomes before and after treatment. Multivariate linear regression analysis was performed using the backward stepwise method to adjust for variables that might influence the outcomes. The independent variables included age, sex, tumor location, preoperative SINS, fusion level, CTJ cross, and rod type. The dependent variable was the change in the segmental Cobb angle (Postoperative − last follow-up), which indicates kyphosis progression. The statistical significance level was set at p < 0.05 (2-tailed).
Baseline demographic data are presented in Table 1. A significant predominance of male patients was observed in the 3.5-mm rod group (p = 0.016). Additionally, the number of patients with metastases at the C7, T1, and T2 levels was higher in the 5.5-mm rod groups (N = 30 in the 5.5-mm rod group vs. N = 6 in the 3.5-mm rod group, p < 0.001), requiring significantly more CTJ crossing instrumentation (N= 31 in the 5.5-mm rod group vs. N= 7 in the 3.5-mm rod group, p < 0.001) (Fig. 3).
The SINS was also significantly worse in the 5.5-mm rod group than in the 3.5-mm rod group (14.2 ± 1.7 vs. 12.4 ± 2.7, p = 0.004).
Surgical details are presented in Table 2. No significant differences in the number of instrumented levels, postoperative revision, complications, instrument pullout, and fracture were observed between the 2 groups. However, in the 3.5-mm rod group, significantly more cases of index vertebral collapse occurred during the follow-up (4 cases, p = 0.021), and the change in the segmental Cobb angle was greater (-1.1 ± 2.1 in the 5.5-mm rod group vs. -7.4 ± 10.7 in the 3.5-mm rod group, p = 0.011) (Figs. 1-2).
Changes in clinical outcomes are presented in Table 3. Although no significant differences in the SOSGOQ and ECOG scores were observed between the 2 groups, neck pain reduction was significantly greater in the 5.5-mm rod group than in the 3.5-mm rod group (postoperative–preoperative: -6.7 ± 2.6 in the 5.5-mm rod group vs. -3.3 ± 3.2 in the 3.5-mm rod group, p = 0.001).
Multivariate linear regression analysis also showed that only rod diameter was a significant variable (p = 0.034) associated with kyphotic progression after fusion surgery (Table 4).
The primary goals of surgery for MCST are to relieve pain, slow neurological deterioration, and maintain spinal stability. Metastatic cervical spinal surgery involves neural decompression and stabilization using screws and rods [3,5,6,12,14]. Although the gold standard for treating MCSTs is corpectomy, followed by graft insertion and plate fixation, via the anterior approach, a paradigm shift has caused the preferential selection of the posterior approach because of its biomechanical superiority. This is influenced more than ever by the widespread use of CPS and technological advances in spinal navigation [3,5,7,12-14].
We have illustrated the safety and effectiveness of CPS in managing various complex spinal conditions, such as cervical spinal tumors, even when using the free-hand technique [3,15-17,19-21,23]. After safely adjusting to the free-hand technique, we could avoid mismatches between the navigation-guided images and the real rotated vertebral body when using navigation during CPS [19,23]. Although these advanced image-guided techniques have improved the accuracy of CPS, they have also increased the costs and operative times. Additionally, the operating room has become cumbersome, and surgeons relying on these technologies risk losing surgical skills and experience [15]. However, considering that MCST surgery is frequently performed under emergency conditions, our free-hand technique-based CPS experience has been extremely beneficial.
A previous study reported that the posterior approach for MCST was sufficient, even with lateral mass screw placement (LMS) [7]. However, LMS requires longer fixation levels than CPS to avoid instrumentation failure. Another study showed that the combination of the anterior and posterior approaches led to better outcomes and more perioperative complications [9]. They also used LMS, indicating that LMS should be performed with the additional anterior approach to achieve reasonable stability. Despite the occurrence of four index vertebral collapses in the 3.5-mm rod group, no patient exhibited implant pullout or fracture, probably because we used CPS. This contrasts with the results of previous studies where LMS was used and such complications were reported [7,13]. Notably, 2 types of CPS (i.e., Vertex or Legacy) can maintain initial pullout strength; however, 5.5-mm rods can only sustain postoperative alignment and reduction, in line with previous reports [22]. This result suggests that the use of four 3.5-mm rods and CPS can sustain the initial reduction although we did not use the aforementioned combination. Although a study reported that nearly 2.8% of hardware failure cases required revision surgery, our series had none [25]. Nonetheless, reduction loss at the last follow-up was significantly greater in the 3.5-mm rod group (-7.4 ± 10.7) than in the 5.5-mm rod group (-1.1 ± 2.1), with a p-value of 0.011, although no screw-related complications occurred in either group. Furthermore, multivariate linear regression analysis showed that the type of rod (5.5 mm vs. 3.5 mm) was the only significant variable affecting reduction loss. These results suggest that 5.5-mm rods can effectively resist the forward bending momentum forces that cause reduction loss.
The CTJ is a biomechanically challenging region for spinal surgery and one of the major risk factors for poor surgical outcomes in MCSTs [8,10-13]. Interestingly, although the 5.5-mm rod group had significantly more lesions located at the CTJ—a region more unstable than C3–6—with a higher mean SINS than the 3.5-mm rod group, the postoperative collapse rate was significantly lower in the 5.5-mm rod group than in the 3.5-mm rod group [4]. Considering that no significant differences in the number of fixation levels and the presence of anterior reconstruction were observed between the 2 groups, 5.5-mm rods could be regarded as strong contributing stabilizers in avoiding postoperative collapse.
Nearly 80% of our patient population was fixed in less than four levels, compared with previous studies in which longer-level fixations were required [5,7-9,13,25]. Longer instrumentation of more than 7 levels was highly associated with instrumentation failure [25]. Our surgical policy in this MCST palliative situation is to adopt minimally short-segment fixation with the decompression of clinically significant compressive lesions, which is also used as a single surgeon’s policy [3].
Traditionally, the consensus was instrumentation of 2 levels above and 2 below with tumor decompression [25,26]. However, considering the development of radiation and chemotherapy, surgeons have a greater chance of encountering patients without an appropriate fixation point who are not traditional surgical candidates because oncologists request surgery more frequently. In this situation, if the surgeon attempts to determine a more stable fixation point, longer-level instrumentation is inevitable, resulting in more complications [25-27]. This paradigm shift made reducing the instrumentation levels and long incision-associated complications and conducting a short-segment fusion for MCST and thoracic spinal metastatic tumors difficult, resulting in our entire practice pattern [23,25,26,28].
In the 5.5-mm rod group, 3 minor (dura tears) and 1 major (i.e., deep wound infection) complications occurred, which required revision surgery, with an overall complication rate of 14.2%. In the 3.5-mm rod group, 2 major (incomplete decompression and cage dislodgement) and 1 minor (dura tear) complications occurred, with an overall complication rate of 13.1%. No vertebral artery injury or CPS-related complications occurred in either group. In this study, the overall complication rate was lower than that in previous studies [26,27].
According to previous studies, most patients experience a loss in kyphotic reduction even after fusion surgery, leading to neck pain [24,29]. Rajshekhar et al. [24] reported a reduction loss of > 10° in 50.5% of patients over an average follow-up duration of 22.2 months; however, in our study, only 4 patients (17.4%) in the 3.5-mm rod group showed a reduction loss of > 10°, which we defined as collapse. This discrepancy may be attributed to the shorter survival duration of patients with MCST [24]. Furthermore, we believe that the more pronounced kyphotic reduction losses and collapses in the 3.5-mm rod group than those in the 5.5-mm rod group are associated with increased neck pain.
This study has several limitations. This was a retrospective analysis that involved a small number of cases: a large proportion in the 3.5-mm rod groups was at a relatively early phase, whereas a large proportion in the 5.5-mm rod group was at a relatively late phase. However, these findings have been advanced throughout the struggle to overcome the frequently occurring mechanical failure of MCSTs due to their biomechanical fragile nature; therefore, these findings are clinically meaningful.
Additionally, we did not analyze the effects of screw diameter in this study, which may be an important factor for construct durability. Nonetheless, in our study, no instrument failure occurred, including screw pull out or screw fracture, regardless of the screw diameter. A systematic review also reported a significantly low screw-related complication rate in CPS placement [30]. Therefore, we believe that once a CPS is properly inserted, instrument failure is very low, regardless of the screw diameter.
In this study, 5.5-mm rods significantly reduced index vertebral collapse after MCST posterior fusion surgery using CPS, although no screw pullout and fracture occurred in either the 3.5-mm or 5.5-mm rod group. This result indicates that 5.5-mm rods can successfully resist forward bending momentum that can develop after MCST posterior fusion surgery. Furthermore, 5.5-mm rods showed complete biomechanical stability, even in the CTJ-crossed instrumentation for MCSTs.
NOTES
Fig. 1.
(A) A 57-year-old male patient with hepatocellular carcinoma metastatic spinal cord compression on the C2 vertebra underwent decompression and fixation from C1 to C3 using the Vertex pedicle screw system (Medtronic Sofamor Danek, Memphis, TN, USA) and 3.5-mm rods. After 7 months, a reduction loss of 11° was noted. (B) A 43-year-old male patient with lung cancer metastatic spinal cord compression on the C2 vertebra underwent decompression and fixation from C1 to C3 using the Legacy pedicle screw system (Medtronic Sofamor Danek) and 5.5-mm rods. After 3 months, no change in the immediate postoperative alignment was observed. (C) A 45-year-old female patient with breast cancer metastatic spinal cord compression and kyphosis on the C2 vertebra underwent decompression, correction, and fixation from C1 to C3 using the Legacy system and 5.5-mm rods. After 1 year, the immediate postoperative alignment was well maintained.

Fig. 2.
(A) A 67-year-old male patient with thymus cancer metastatic spinal cord compression on the C5 vertebra underwent decompression, fixation, and anterior support from the C4 to C6 vertebrae using the Vertex pedicle screw system (Medtronic Sofamor Danek, Memphis, TN, USA) and 3.5-mm rods. After 1.5 years, a reduction loss of 13°–31° was observed; however, revision surgery was unnecessary because the patient’s neck pain was not severe (preoperative Numeric Rating Scale [NRS], 7 vs. postoperative NRS, 4), and no neurological deterioration was observed. (B) A 60-year-old female patient with cervical cancer metastatic spinal cord compression with kyphosis on the C6 vertebra underwent decompression, reduction, and fixation surgery from the C5 to C7 vertebrae using the Legacy pedicle screw system (Medtronic Sofamor Danek) and 5.5-mm rods. On the C7 vertebra, 1 cervical pedicle screw and 1 lamina screw were used. After 1 month, the immediate postoperative alignment was unchanged.

Fig. 3.
(A) A 67-year-old male patient with lung cancer metastatic spinal cord compression on the T1–2 vertebra underwent decompression, anterior support, and relatively long-level fixation surgery from the C5 to T5 vertebrae using the Vertex pedicle screw system (Medtronic Sofamor Danek, Memphis, TN, USA) and Legacy pedicle screw system (Medtronic Sofamor Danek) with 3.5- and 5.5-mm hybrid tapered rods. After 1.5 years, the immediate postoperative alignment was well maintained. (B) A 77-year-old male patient with prostate cancer metastatic spinal cord compression on the T1 vertebrae underwent decompression and fixation surgery from the C7 to T2 vertebrae using the Legacy system and 5.5-mm rods. After 2 years, the immediate postoperative alignment was well maintained, and computed tomography sagittal imaging showed complete fusion.
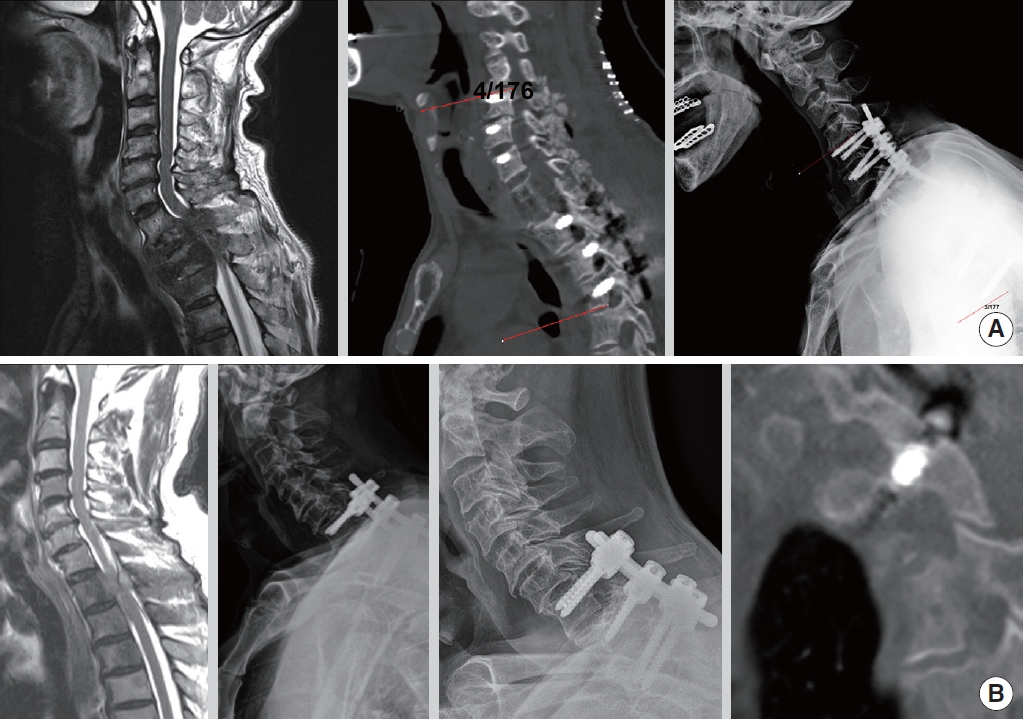
Table 1.
Demographic and clinical characteristics
| Characteristic | 5.5-mm rod group (N=35) | 3.5-mm rod group (N=23) | p-value |
|---|---|---|---|
| Age (yr) | 58.3 ± 13.1 (24–77) | 62.7 ± 12.2 (26–80) | 0.208 |
| Sex | 0.016* | ||
| Male | 18 (51.4) | 19 (82.6) | |
| Female | 17 (48.6) | 4 (17.4) | |
| Histology | 0.109 | ||
| Lung | 8 (22.9) | 6 (26.1) | |
| Pancreas | 5 (14.3) | 0 (0) | |
| Liver | 4 (11.4) | 4 (17.4) | |
| Renal | 4 (11.4) | 0 (0) | |
| Breast | 3 (8.6) | 0 (0) | |
| Colon | 2 (5.7) | 0 (0) | |
| GB | 0 (0) | 2 (8.7) | |
| Stomach | 1 (2.9) | 1 (4.3) | |
| Prostate | 1 (2.9) | 1 (4.3) | |
| MM | 1 (2.9) | 1 (4.3) | |
| Other† | 6 (17.1) | 8 (34.8) | |
| Tumor location | < 0.001*** | ||
| C2 | 3 (8.6) | 4 (17.4) | |
| C3–6 | 2 (5.7) | 13 (56.5) | |
| C7–T2 | 30 (85.7) | 6 (26.1) | |
| Revision | 0.270 | ||
| Yes | 3 (8.6) | 0 (0) | |
| No | 32 (91.4) | 23 (100) | |
| Anterior column support | 0.513 | ||
| Yes | 6 (17.1) | 6 (26.1) | |
| No | 29 (82.9) | 17 (73.9) | |
| Preoperative SINS | 14.2 ± 1.7 (11–18) | 12.4 ± 2.7 (8–18) | 0.004** |
| CTJ cross | < 0.001*** | ||
| Yes | 31 (88.6) | 7 (30.4) | |
| No | 4 (11.4) | 16 (69.6) |
Table 2.
Comparison of surgical details, complications, and segmental Cobb angle changes between the 5.5-mm and 3.5-mm rod groups
| Variable | 5.5-mm rod group (N=35) | 3.5-mm rod group (N=23) | p-value |
|---|---|---|---|
| No. of instrumented levels | 0.248 | ||
| 2 | 12 (34.3) | 12 (52.2) | |
| 3 | 6 (17.1) | 5 (21.7) | |
| 4 | 8 (22.9) | 4 (17.4) | |
| 5 | 6 (17.1) | 0 (0) | |
| 6 | 1 (2.9) | 0 (0) | |
| 7 | 2 (5.7) | 2 (8.7) | |
| Postoperative revision surgery | 0.556 | ||
| Yes | 1 (2.9) | 2 (8.7) | |
| No | 34 (97.1) | 21 (91.3) | |
| Complications | 1.000 | ||
| Yes | 5 (14.3) | 3 (13.0) | |
| Major† | 1 | 2 | |
| Minor‡ | 4 | 1 | |
| No | 30 (85.7) | 20 (87.0) | |
| Instrument pullout or fracture | 1.000 | ||
| Yes | 0 (0) | 0 (0) | |
| No | 35 (100) | 23 (100) | |
| Δ Segmental Cobb angle | |||
| Postoperative–preoperative | 2.2 ± 12.3 | 6.5 ± 12.9 | 0.205 |
| < -10 | 4 (11.4) | 2 (8.7) | |
| -10 to 0 | 13 (37.1) | 7 (30.4) | |
| 1 to 10 | 12 (34.3) | 7 (30.4) | |
| > 10 | 6 (17.1) | 7 (30.4) | |
| Last follow-up–postoperative | -1.1 ± 2.1 | -7.4 ± 10.7 | 0.011* |
| < -10 | 0 (0) | 4 (17.4) | |
| -10 to 0 | 35 (100) | 19 (82.6) | |
| Follow-up duration (mo) | 12.6 ± 14.3 | 11.8 ± 16.5 | 0.849 |
Table 3.
Comparison of clinical outcomes and performance status between the 5.5-mm and 3.5-mm rod groups
| Variable | 5.5-mm rod group (N=35) | 3.5-mm rod group (N=23) | p-value |
|---|---|---|---|
| Neck NRS | |||
| Preoperative | 7.9 ± 2.5 | 5.9 ± 3.1 | < 0.001*** |
| Postoperative | 1.1 ± 1.4 | 2.5 ± 1.6 | 0.005* |
| ΔNRS‡ | -6.7 ± 2.6 | -3.3 ± 3.2 | 0.001** |
| SOSGOQ | |||
| Preoperative | 20.1 ± 15.4 | 34.3 ± 34.8 | 0.373† |
| Postoperative | 70.1 ± 17.2 | 57.5 ± 13.7 | 0.103† |
| ECOG PS§ | |||
| Preoperative | 1.000† | ||
| 0–1 | 8 (72.7) | 8 (80.0) | |
| 2–4 | 3 (27.3) | 2 (20.0) | |
| Postoperative | 0.408† | ||
| 0–1 | 10 (66.7) | 10 (83.3) | |
| 2–4 | 5 (33.3) | 2 (16.7) | |
| Follow-up duration (mo) | 12.6 ± 14.3 | 11.8 ± 16.5 | 0.849 |
Values are presented as mean±standard deviation or number (%).
NRS, Numeric Rating Scale; SOSGOQ, Spine Oncology Study Group Outcomes Questionnaire; ECOG PS, Eastern Cooperative Oncology Group performance status scale.
† As this is a retrospective study, some data on these items are missing. Nonparametric analyses were performed on data that were not normally distributed.
§ ECOG-PS is a nearly 50-year-old tool used in both research and oncology treatment. It is strongly associated with survival in advanced cancer. Studies have divided patients into two groups based on ECOG-PS 2 or higher to evaluate outcome measures; therefore, we analyzed the data using this criterion in this study.
Table 4.
Multivariate linear regression of the change in the segmental Cobb angle between postoperative and last followup, indicating kyphosis progression
| Variable | β | 95% CI | SE | p-value |
|---|---|---|---|---|
| Group | ||||
| 3.5-mm rod | Reference | |||
| 5.5-mm rod | -4.82 | -9.28 to -0.37 | 2.22 | 0.034* |
| CTJ cross | ||||
| No | Reference | |||
| Yes | -3.25 | -7.83 to 1.34 | 2.29 | 0.161 |
REFERENCES
1. Maccauro G, Spinelli MS, Mauro S, et al. Physiopathology of spine metastasis. Int J Surg Oncol 2011;2011:107969.




3. Shin HK, Kim M, Lee S, et al. Surgical strategy for metastatic spinal tumor patients with surgically challenging situation. Medicine (Baltimore) 2022;101:e29560.



4. Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the spine oncology study group. Spine (Phila Pa 1976) 2010;35:E1221-9.

5. Jenis LG, Dunn EJ, An HS. Metastatic disease of the cervical spine: a review. Clin Orthop Relat Res 1999;359:89-103.
6. Sciubba DM, Gokaslan ZL. Are patients satisfied after surgery for metastatic spine disease? Spine J 2010;10:63-5.

7. Gallazzi E, Cannavò L, Perrucchini GG, et al. Is the posterior-only approach sufficient for treating cervical spine metastases? The evidence from a case series. World Neurosurg 2019;122:e783-9.

8. Kanda Y, Kakutani K, Sakai Y, et al. Surgical outcomes and risk factors for poor outcomes in patients with cervical spine metastasis: a prospective study. J Orthop Surg Res 2021;16:1-9.


9. Luksanapruksa P, Santipas B, Rajinda P, et al. Postoperative outcomes of subaxial cervical spine metastasis: comparison among the anterior, posterior, and combined approaches. J Bone Oncol 2022;34:100424.

10. Balestrino A, Gondar R, Jannelli G, et al. Surgical challenges in posterior cervicothoracic junction instrumentation. Neurosurg Rev 2021;44:3447-58.


11. Bueff HU, Lotz JC, Colliou OK, et al. Instrumentation of the cervicothoracic junction after destabilization. Spine (Phila Pa 1976) 1995;20:1789-92.

12. Hubertus V, Gempt J, Mariño M, et al. Surgical management of spinal metastases involving the cervicothoracic junction: results of a multicenter. Neurosurg Focus 2021;50:E7.
13. Chakravarthy VB, Hussain I, Laufer I, et al. Cervicothoracic junction instrumentation strategies following separation surgery for spinal metastases. J Neurosurg Spine 2023;38:473-80.

14. Oda I, Abumi K, Ito M, et al. Palliative spinal reconstruction using cervical pedicle screws for metastatic lesions of the spine: a retrospective analysis of 32 cases. Spine (Phila Pa 1976) 2006;31:1439-44.

15. Park JH, Jeon SR, Roh SW, et al. The safety and accuracy of freehand pedicle screw placement in the subaxial cervical spine: a series of 45 consecutive patients. Spine (Phila Pa 1976) 2014;39:280-5.

16. Park JH, Roh SW, Rhim SC. A single-stage posterior approach with open reduction and pedicle screw fixation in subaxial cervical facet dislocations. J Neurosurg Spine 2015;23:35-41.


17. Chon H, Park JH. Cervical vertebral body fracture with ankylosing spondylitis treated with cervical pedicle screw: a fracture body overlapping reduction technique. J Clin Neurosci 2017;41:150-3.


18. Lee S, Seo J, Lee MK, et al. Widening of the safe trajectory range during subaxial cervical pedicle screw placement: advantages of a curved pedicle probe and laterally located starting point without creating a funnel-shaped hole. J Neurosurg Spine 2017;27:150-7.


19. Heo Y, Lee SB, Lee BJ, et al. The learning curve of subaxial cervical pedicle screw placement: how can we avoid neurovascular complications in the initial period? Oper Neurosurg (Hagerstown) 2019;17:603-7.



20. Kim MW, Lee SB, Park JH. Cervical spondyloptosis successfully treated with only posterior short segment fusion using cervical pedicle screw fixation. Neurol Med Chir (Tokyo) 2019;59:33-8.



21. Jung YG, Jung SK, Lee BJ, et al. The subaxial cervical pedicle screw for cervical spine diseases: the review of technical developments and complication avoidance. Neurol Med Chir (Tokyo) 2020;60:231-43.



22. Lee S, Cho DC, Roh SW, et al. Cervical alignment following posterior cervical fusion surgery: cervical pedicle screw versus lateral mass screw fixation. Spine (Phila Pa 1976) 2021;46:E576-83.

23. Shin HK, Jeon SR, Roh SW, et al. Benefits and pitfalls of O-Arm navigation in cervical pedicle screw. World Neurosurg 2022;159:e460-5.


24. Rajshekhar V, Arunkumar MJ, Kumar SS. Changes in cervical spine curvature after uninstrumented one- and two-level corpectomy in patients with spondylotic myelopathy. Neurosurgery 2003;52:799-805.



25. Amankulor NM, Xu R, Iorgulescu JB, et al. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J 2014;14:1850-9.


26. Newman WC, Bilsky MH. Fifty‐year history of the evolution of spinal metastatic disease management. J Surg Oncol 2022;126:913-20.



27. Bilsky MH, Laufer I, Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine (Phila Pa 1976) 2009;34(22 Suppl):S101-7.


28. Moussazadeh N, Rubin DG, McLaughlin L, et al. Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J 2015;15:1609-17.






























