 |
 |
- Search
| Neurospine > Volume 21(1); 2024 > Article |
|
|
Abstract
Objective
To evaluate the global practice pattern of wound dressing use after lumbar fusion for degenerative conditions.
Methods
A survey issued by AO Spine Knowledge Forums Deformity and Degenerative was sent out to AO Spine members. The type of postoperative dressing employed, timing of initial dressing removal, and type of subsequent dressing applied were investigated. Differences in the type of surgery and regional distribution of surgeons’ preferences were analyzed.
Results
Right following surgery, 60.6% utilized a dry dressing, 23.2% a plastic occlusive dressing, 5.7% glue, 6% a combination of glue and polyester mesh, 2.6% a wound vacuum, and 1.2% other dressings. The initial dressing was removed on postoperative day 1 (11.6%), 2 (39.2%), 3 (20.3%), 4 (1.7%), 5 (4.3%), 6 (0.4%), 7 or later (12.5%), or depending on drain removal (9.9%). Following initial dressing removal, 75.9% applied a dry dressing, 17.7% a plastic occlusive dressing, and 1.3% glue, while 12.1% used no dressing. The use of no additional coverage after initial dressing removal was significantly associated with a later dressing change (p < 0.001). Significant differences emerged after comparing dressing management among different AO Spine regions (p < 0.001).
Conclusion
Most spine surgeons utilized a dry or plastic occlusive dressing initially applied after surgery. The first dressing was more frequently changed during the first 3 postoperative days and replaced with the same type of dressing. While dressing policies tended not to vary according to the type of surgery, regional differences suggest that actual practice may be based on personal experience rather than available evidence.
Surgical site infection (SSI) is a major cause of morbidity following spine surgery, with an increased risk of pseudoarthrosis, deformity, and even death [1]. The reported incidence of SSIs following posterior lumbar spine fusion ranges between 2% and 20% [2], with higher rates in patients affected by diabetes and obesity, >3-hour-long surgeries, and when using posterior approaches [3]. Overall, SSIs pose a tremendous clinical and socioeconomic burden on both patients and healthcare facilities [4]. Indeed, SSIs are one of the most common reasons for hospital readmission and often require extensive intravenous antibiotic administration, revision surgery, and prolonged hospital stay [5]. Therefore, considering their devastating outcomes, it is essential to strictly apply preventive measures preoperatively, intraoperatively, and postoperatively.
Accurate wound care is fundamental to lowering the rate of postsurgical SSIs. The application of sterile wound dressings has the objective of preserving the sterile environment of the operating room, absorbing any wound drainage, and even locally delivering antiseptic treatments to prevent wound infection [6]. Furthermore, wound dressings also serve as protection against pressure, friction and irritation, which are common due to prolonged supine position after posterior lumbar spine surgery [7]. To date, several different dressing options are available for postoperative wound care. These include traditional dry dressings, plastic occlusive dressings, and more advanced solutions, such as silver-impregnated dressings, characterized by inherent antiseptic properties. Additionally, the utilization of skin glue has been increasingly reported due to ease of application and avoiding the use of additional dressings. In selected cases, especially when wound healing complications are expected (e.g., revision surgery, multilevel procedures), incisional wound vacuum may also be utilized. Although several dressing protocols and materials are available, there is no consensus regarding the specific type of dressing to be used or the timing of dressing removal. Indeed, indications concerning dressing policy are fragmentary and often related to local policies rather than international guidelines [8].
The aim of this study was to evaluate current worldwide dressing protocols following lumbar spine surgery for degenerative conditions. We have analyzed the data extracted from a global online survey conducted by AO Spine to describe the most common dressing strategies and comparing them in terms of different types of surgery performed and regional variability. More specifically, the type of dressing usually employed, timing of initial dressing removal and type of subsequent dressing applied were investigated.
An online questionnaire focused on perioperative outcomes and aspects of spine surgery was formulated by AO Spine Knowledge Forum Degenerative and Deformity working groups. No formal Institutional Review Board approval was needed for this study. The survey was sent out by email to all AO Spine users and members between March 3 and March 22, 2022. All the participants signed a digital informed consent and agreed on the use of their anonymized answers for research purposes. Answers from spine surgeons performing ≥ 10 cases per year of one or more of the following procedures were retrospectively analyzed: (A) long fusion (> 5 levels) for adult spine deformity patients extending to the pelvis; (B) long fusion (> 5 levels) for adult spine deformity patients not extending to the pelvis; (C) open 1- or 2-level fusion for adult lumbar degenerative pathologies; (D) minimally invasive surgery (MIS) 1- or 2-level fusion for adult lumbar degenerative pathologies; (E) open 3- to 5-level fusion for adult lumbar degenerative pathologies. For this study, only answers from surgeons performing lumbar spine surgery for degenerative conditions (C-E) were included.
Retrieved data included participants’ country and AO Spine region of practice, sex, age, years of practice in spine surgery, specialty, practice setting and information about spine surgery fellowship were retrieved, as well as surgical case volume. Participants were asked if performing ≥ 10 cases of one or more of the following procedures for adult lumbar degenerative disorders: open 1- or 2-level fusion; MIS 1- or 2-level fusion; open 3- to 5-level fusion. With regards to postoperative dressing management, the type of postoperative dressing usually employed, timing of initial dressing removal and type of subsequent dressing applied were investigated. As surgeons could choose more than one option, multiple data entries for the same participant were also considered. The questionnaire is available as Supplementary Material.
Categorical data were shown as absolute (n) and relative (%) frequencies. Statistical analysis was performed using the chi-square test. To ensure test validity, data groups were opportunely combined when values were lower than 1 and/or when more than 20% of the values were lower than 5. Logistic regression was performed to evaluate the relationship between the use of dressing versus no dressing following the initial dressing change (independent variable) and the timing of dressing change (postoperative day, dependent variable). Furthermore, a multiple logistic regression model was built to investigate the associations between MIS and open surgery (dependent variables) and work setting (private/public/academic), type of initial dressing, postoperative day of dressing change and type of second dressing. Open surgery was considered a negative outcome (encoded as “0”) and MIS as a positive outcome (encoded as “1”). The most frequent categories per each independent variable were selected as reference levels (work setting: academic; type of initial dressing: dry dressing; postoperative day of dressing change: 2; type of second dressing: dry dressing). Similarly, the associations between surgeons’ years of experience (< 15 and > 15) and type of initial dressing, postoperative day of dressing change and type of second dressing were investigated. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for each reference category. An area under the curve (AUC)> 0.7 was considered acceptable. Statistical significance was set at p < 0.05. Formal analysis was conducted using Prism ver. 9.5.1 (GraphPad Software Inc., La Jolla, CA, USA).
A total of 354 spine surgeons responded to the questionnaire and 280 completed it (79.1%). Among these, 261 routinely performed lumbar degenerative spine surgery and were thus analyzed (n = 261; Fig. 1). The responders were distributed among North America (19.1%), Latin America (14.2%), Europe & Southern Africa (36.0%), Middle East & Northern Africa (8.0%), and Asia-Pacific (22.6%) regions. Most surgeons were male (99.2%), aged between 35 and 44 (42.1%), and in practice for at least 5 years (83.9%). Most participants were orthopaedic surgeons (75.1%), 45.6% practiced in an academic hospital, 26.4% in a public hospital, and 25.7% in a private setting. The majority completed a spine surgery fellowship (63.2%) and operated between 50 and 150 cases per year (47.9%). Among these, 233 (89.3%) performed 1- and 2-level open fusion surgeries, 112 (42.9%) performed 1- and 2-level MIS fusion surgeries, and 189 (72.4%) performed 3- to 5-level open fusion surgeries for degenerative pathologies. Right following surgery, 60.6% utilized a dry dressing, 23.2% a plastic occlusive dressing, 5.7% applied glue, 6% used a combination of glue and polyester mesh (DERMABOND PRINEO Skin Closure System, Ethicon, Johnson & Johnson; Raritan, NJ, USA), 2.6% an incisional wound vacuum, and 1.2% other dressings. No postoperative dressing was used by 0.6% of responders (Fig. 2A). The initial dressing was removed either on postoperative day 1 (11.7%), 2 (39.2%), 3 (20.3%), 4 (1.7%), 5 (4.3%), 6 (0.4%), 7 or later (12.5%), or depending on drain removal (9.9%; Fig. 2B). Following initial dressing removal, 75.9% applied a dry dressing, 17.7% a plastic occlusive dressing, and 1.3% glue, while 12.1% used no dressing (Fig. 2C). No statistical significance was found when comparing the type of initial dressing (p = 0.792) (Table 1), timing of initial dressing change (p = 0.926) (Table 2), and type of subsequent dressing (p = 0.933) (Table 3) in terms of different surgical procedures performed. However, the use of no dressing following dressing change was significantly associated with a later removal of the initial dressing (p < 0.001; 95% CI, 2.527–3.701; AUC, 0.73) (Fig. 3). Furthermore, we built a multiple logistic regression model to investigate the association between MIS or open surgery and the other investigated variables (work setting, type of initial dressing, timing of dressing change, and type of second dressing). Intriguingly, while MIS was significantly associated with the use of a plastic occlusive dressing as the initial wound cover (OR, 1.79; 95% CI, 1.04–3.05; p = 0.034), no additional statistically significant relationships were found. Similarly, no significant association was found between surgeons’ years of experience and type of initial dressing, timing of dressing change, and type of second dressing.
Interestingly, statistically significant differences were found when comparing postoperative dressing protocols among different AO Spine regions in terms of type of initial dressing (p < 0.001) (Table 4), timing of initial dressing removal (p < 0.001) (Table 5), and type of subsequent dressing used (p < 0.001) (Table 6). Dry dressing was the most utilized in all 5 regions (Asia-Pacific, 58.4%; Europe & Southern Africa, 74.2%; Latina America, 59.8%; Middle East & Northern Africa, 89.8%; North America, 47.1%), followed by plastic occlusive dressing (Asia-Pacific, 32.0%; Europe & Southern Africa, 18.1%; Latina America, 28.3%; Middle East & Northern Africa, 10.3%; North America, 27.5%). Intriguingly, glue was used mostly in North America (11.6%) and Europe & Southern Africa (7.2%), while the combination of glue and polyester mesh was mainly employed in North America (13.8%) and Asia-Pacific (7.2%) regions (Fig. 4A). In terms of timing of dressing change, postoperative days 2 and 3 were the most common time points among all 5 regions (Fig. 4B). While approximately 20% of surgeons in Europe & Southern Africa and Latin America changed the initial dressing as soon as postoperative day 1, less than 6% did so in Asia-Pacific and North America regions. On the other hand, approximately 25% of respondents from the Asia-Pacific region reported removing the initial dressing on day 7 or later. When analyzing data regarding the type of dressing applied following the change of the initial dressing, dry dressing was again the most common among all practitioners (Fig. 4C). While only a few responders reported using glue, plastic occlusive dressing was the most popular choice in respondents from Asia-Pacific (38%) and Latin America (26.7%). Interestingly, whilst one-third of surgeons from North America used no dressing following removal of the initial dressing, only 3.9% of participants from Europe & Southern Africa reported leaving the surgical wound uncovered.
To our knowledge, this is the first cross-sectional study to specifically investigate different wound dressing protocols in lumbar spine degenerative surgery across AO Spine practitioners worldwide. According to our results, dry and plastic occlusive dressings were the most utilized, and postoperative dressing was changed more frequently during the first 3 days after surgery. Subsequently, a new dry or plastic occlusive dressing was applied, either the wound was left uncovered. More specifically, the use of a subsequent dressing was more likely when respondents changed the initial dressing during the first postoperative days. On the other hand, when the initial dressing was kept in place for a longer time, a higher chance of leaving the surgical wound uncovered was reported. In addition, MIS surgical procedures were significantly associated with the use of a plastic occlusive dressing as the initial wound cover.
While no evidence summary for the prevention of SSIs in lumbar spine surgery is available at present, both the World Health Organization [9] and Centers for Disease Control and Prevention [10] have released general guidelines on wound care and dressing management. Overall, in accordance with a Cochrane review updated in 2016 [11], it is unclear whether any specific dressing is superior to others in reducing the risk of SSI and even if leaving the surgical wound exposed affects SSI risk compared to the use of any dressing. As reported, the high degree of uncertainty is mostly due to the low quality of available evidence and high risk of bias of included studies. Therefore, the most appropriate type of dressing should be selected based on dressing costs and surgeon preference. This was also confirmed by a recent evidence summary on prophylactic postoperative measures to reduce SSIs after spine surgery [12].
While the use of dry dressings is usually preferred for their capacity to absorb wound drainage and low cost, plastic transparent occlusive dressings may have the advantage of monitoring the wound without the need to change the dressing thus preventing outside contamination [13,14]. Furthermore, specific dressing strategies have also been developed to directly exert antiseptic effects or promote surgical wound healing. These include skin glue, silver-impregnated dressings, and incisional wound vacuum. Modern skin glue, mainly composed of glue, has the ability to provide the wound with a mechanical barrier as well as bactericidal properties against gram-positive bacteria [15]. In a recent systematic review from Tan et al. [16], cyanoacrylate dermal closure was associated with significantly low rates of SSIs following both lumbar microdiscectomy and laminectomy (0.4% and 1.8%, respectively). However, included studies were mainly composed of case series, thus substantially impacting the reliability of data. Nonetheless, the use of skin glue was reported by approximately 12% of our survey responders (considering both glue alone and the combination of glue with a polyester mesh). Interestingly, the use of skin glue was mostly employed by North American surgeons in our cohort. Due to the antibacterial activity of silver against both gram-positive and gram-negative microorganisms, the use of silver-impregnated dressings has already been successfully reported in preventing SSIs in lumbar degenerative surgery. Indeed, Epstein [6] showed that the use of silver-impregnated dressings reduced the rate of SSI to 0% compared to the use of dry dressings in 106 patients undergoing lumbar multilevel laminectomy and instrumented fusion. However, due to uncertain benefits and harms (including allergic reactions and skin irritation), and low quality of available evidence, the use of silver-impregnated dressings over traditional dressings is not recommended by most guidelines [8-10]. A limited number of surgeons from our cohort (2%–5%) also reported the use of incisional wound vacuum as a dressing strategy. By reducing tensile forces and edema, encouraging blood and lymphatic flow around the surgical wound, and promoting exudate draining, negative pressure wound therapy (NPWT) has been previously utilized in spine surgery. In a recent prospective study, Mueller and colleagues demonstrated that NPWT was associated with significantly lower SSI rates compared with standard dressing strategies (3.4% vs. 10.9%) in patients undergoing spine surgery for degenerative disease, deformity, malignancy, and trauma. However, no significant difference was found in subgroup analyses for patients undergoing lumbosacral surgery (9.2% vs. 3%) or surgery for degenerative conditions (1.3% vs. 3.4%) [17]. A similar study conducted by Akhter et al. [18] displayed a significantly lower rate of SSIs in patients undergoing posterior spine fusion surgery treated with NPWT compared to routine dressings (0% vs. 7.1%). However, no subgroup analysis of patients receiving lumbar spine surgery for degenerative pathologies was provided in this study. Although the use of NPWT seems advantageous in “high risk” patients (e.g., posterior open surgery at the cervicothoracic junction, metastatic disease, multiple comorbidities, high-energy trauma, revision surgery, etc.), its routine use to prevent SSIs is generally not recommended [8,9].
Timing of dressing change is widely debated, and no consensus nor guidelines are available [8]. As demonstrated by the notable variability of our survey responses, practitioners were divided among those removing postoperative dressing during the first 3 days after surgery, those waiting up to 7 days, and those changing the dressing at the same time of drain removal (which likely occurred in the first postoperative days). Apparently, there is no scientific evidence in terms of the relationship between SSIs and timing of dressing changes, and most practices depend upon surgeon preference, anecdotal experiences, and individual training. As wound drainage is more likely during the first postoperative days, the removal of dirty dressings is usually performed with the assumption that keeping the wound clean would reduce the risk of SSIs. However, Bains et al. [19] showed that keeping the postoperative dressing in place for 5 days significantly abated SSI rates from 5.5% to 1.1% in posterior lumbar fusion cases. More specifically, the authors advocated the risk of bacterial inoculation of the surgical wound after surgery in the nonsterile environment of hospital wards. However, the actual evidence suggests that there is no consensus regarding the minimal time for which dressings should remain intact to reduce SSI rates in any type of spine surgery [12]. Future studies are needed to formulate evidence-based recommendations for the management of wound dressing in the setting of lumbar degenerative disorders. Moreover, the role of specific advanced wound dressing strategies, such as NPWT and antiseptic dressings, should be further delineated.
This study has some limitations. First, the overall response rate was low, and some regions were significantly less represented than others, thus likely causing response bias and limiting the generalizability of the results to a wider population of practitioners. Second, due to its cross-sectional design, this study cannot impute any causality nor evaluate any change of practice over time. Indeed, the use of different dressing protocols and materials may also depend on factors other than surgeon preference, such as institutional supply. Third, the diverse geographical distribution of dressing protocols may also reflect differences among regional incomes, which were not investigated in this study. In this regard, future studies will need to reach out to a broader, more representative population of practitioners and additionally investigate economic and patient-related factors that may motivate the application of specific dressing protocols.
This study provided an outlook of postoperative dressing management in lumbar spine surgery for degenerative conditions among AO Spine practitioners worldwide. Most respondents used a dry dressing or a plastic occlusive dressing, which was frequently removed during the first 3 postoperative days and replaced with the same type of dressing. Otherwise, postoperative dressing was kept in place for up to 7 days, and then likely removed without being replaced. While dressing protocols did not vary according to the type of surgery, significant regional variability suggests that actual practice is determined by local protocols rather than scientific evidence.
Supplementary Material
Supplementary material can be found via https://doi.org/10.14245/ns.2347168.584.
NOTES
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: AO Spine Knowledge Forum Degenerative; Formal Analysis: LA; Investigation: LA, GV, JT, LS, GBB, SJL, SK, SKC, STY, HJK, MFG, AO Spine Knowledge Forum Degenerative; Methodology: AO Spine Knowledge Forum Degenerative; Writing - Original Draft: LA, GV; Writing - Review & Editing: LA, GV, JT, LS, GBB, VD.
ACKNOWLEDGEMENTS
This study was organized by AO Spine through the AO Spine Knowledge Forum Degenerative and Knowledge Forum Deformity, a focused group of international spine degeneration experts. AO Spine is a clinical division of the AO Foundation, which is an independent medically-guided not-for-profit organization. Study support was provided directly through the AO Spine Research Department.
Fig. 2.
Surgeons’ preferences in terms of type of initial dressing (A), timing of initial dressing change (B), and type of subsequent dressing applied according to different surgical procedures. MIS, minimally invasive surgery; POD, postoperative day.
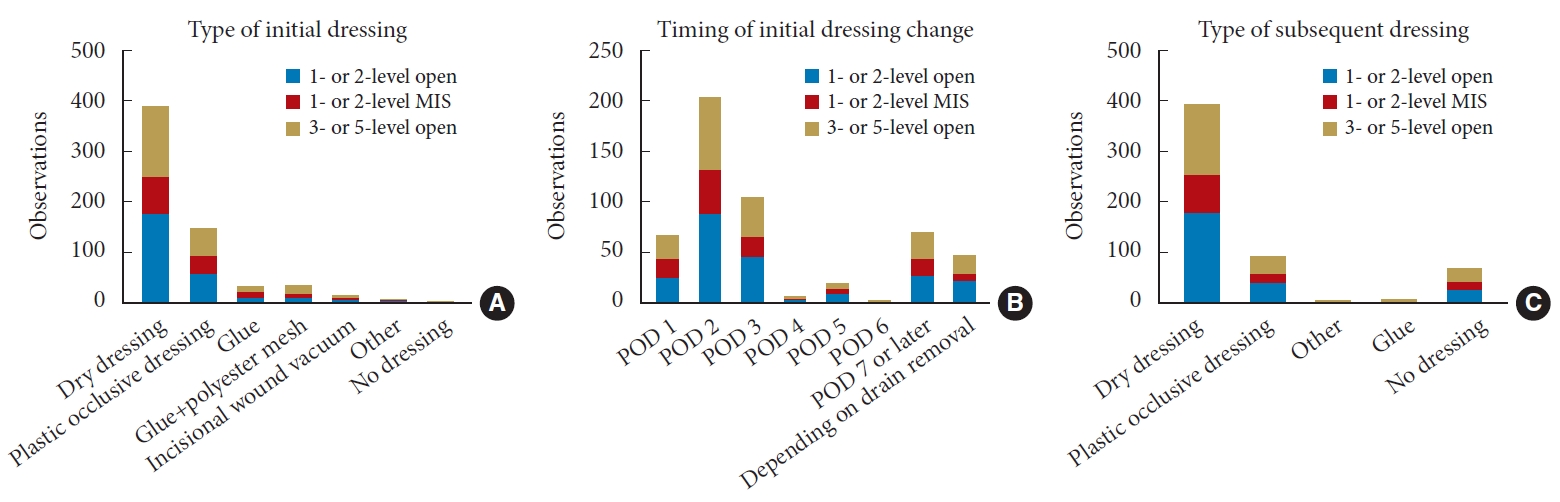
Fig. 3.
Association between timing of initial dressing change and use of a subsequent dressing. POD, postoperative day.
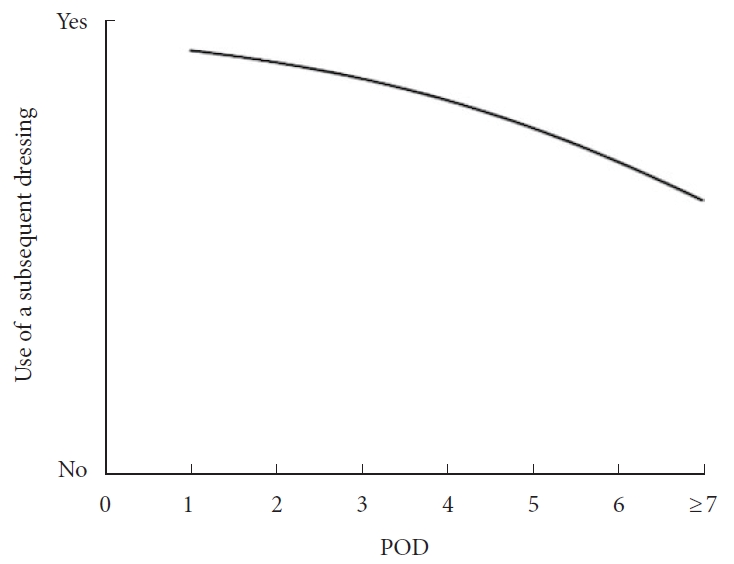
Fig. 4.
Surgeons’ preferences in terms of type of initial dressing (A), timing of initial dressing change (B), and type of subsequent dressing applied according to different AO Spine regions. POD, postoperative day.

Table 1.
Type of initial postoperative dressing according to surgical procedure
Table 2.
Timing of initial dressing change according to surgical procedure
Table 3.
Type of dressing applied following removal of initial postoperative dressing according to surgical procedure
Table 4.
Type of initial postoperative dressing according to different AO Spine regions
Table 5.
Timing of initial dressing change according to different AO Spine regions
Table 6.
Type of dressing applied following removal of initial postoperative dressing according to different AO Spine regions
REFERENCES
1. Casper DS, Zmistowski B, Hollern DA, et al. The effect of postoperative spinal infections on patient mortality. Spine (Phila Pa 1976) 2018;43:223-7.


2. Nota SP, Braun Y, Ring D, et al. Incidence of surgical site infection after spine surgery: what is the impact of the definition of infection? Clin Orthop Relat Res 2015;473:1612-9.



3. Fei Q, Li J, Lin J, et al. Risk factors for surgical site infection after spinal surgery: a meta-analysis. World Neurosurg 2016;95:507-15.


4. Patel H, Khoury H, Girgenti D, et al. Burden of surgical site infections associated with select spine operations and involvement of Staphylococcus aureus. Surg Infect (Larchmt) 2017;18:461-73.



5. Maruo K, Berven SH. Outcome and treatment of postoperative spine surgical site infections: predictors of treatment success and failure. J Orthop Sci 2014;19:398-404.


6. Epstein NE. Do silver-impregnated dressings limit infections after lumbar laminectomy with instrumented fusion? Surg Neurol 2007;68:483-5.


7. Andrew Glennie R, Dea N, Street JT. Dressings and drains in posterior spine surgery and their effect on wound complications. J Clin Neurosci 2015;22:1081-7.


8. Atesok K, Papavassiliou E, Heffernan MJ, et al. Current strategies in prevention of postoperative infections in spine surgery. Global Spine J 2019;10:183-94.




9. World Health Organization. Global guidelines for the prevention of surgical site infection. 2nd edition [Internet]. Geneva (Switzerland), World Health Organization. 2018;[2023 Dec 3]. Available from: https://www.who.int/publications/i/item/9789241550475.
10. Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017;152:784-91.


11. Dumville JC, Gray TA, Walter CJ, et al. Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev 2016;12:CD003091.


12. Tan T, Lee H, Huang MS, et al. Prophylactic postoperative measures to minimize surgical site infections in spine surgery: systematic review and evidence summary. Spine J 2020;20:435-47.


13. O’Brien G, Buckley K, Vanwalleghem G, et al. A multi-centre, prospective, clinical in-market evaluation to assess the performance of Opsite™ Post-Op Visible dressings. Int Wound J 2010;7:329-37.



14. Rocos B, Davidson B, Rabinovitch L, et al. Local contamination is a major cause of early deep wound infections following open posterior lumbosacral fusions. Spine Deform 2023;11:1209-221.



15. Rushbrook JL, White G, Kidger L, et al. The antibacterial effect of 2-octyl cyanoacrylate (Dermabond(R)) skin adhesive. J Infect Prev 2014;15:236-9.


16. Tan T, Rutges J, Marion T, et al. Cyanoacrylate dermal closure in spine surgery: systematic review and pooled analysis. Global Spine J 2019;10:493-8.




17. Mueller KB, D’Antuono M, Patel N, et al. Effect of incisional negative pressure wound therapy vs standard wound dressing on the development of surgical site infection after spinal surgery. Neurosurgery 2021;88:E445-51.






























