- Search
|
|
||
Abstract
Objective
The space available for the spinal cord (SAC) is a measure of spinal cord functional reserve and may vary in different societies. The objective of this study is to measure normal SAC at each subaxial cervical disc level of asymptomatic adult Nigerians and to compare obtained values with published studies worldwide.
Methods
This is a prospective, cross-sectional study using magnetic resonance imaging facility at Memfys Hospital Enugu, from 2012 to 2013. Disc level measurement of midsagittal spinal canal and cord of randomly selected 102 consenting asymptomatic adults, 21 to 50 years. Literature search of related studies worldwide was used to compare with the current study. Analysis was done using inferential and descriptive statistics.
Results
Average SAC values were 4.9±1.4 mm (C3/4), 4.5±1.2 mm (C4/5), 4.6±1.4 mm (C5/6), and 4.9±1.2 mm (C6/7). In 21–30 years group, SAC was 5.4±0.6 mm(C3/4), 4.9±0.6 mm(C4/5), 4.9±0.6 mm(C5/6), and 5.1±0.5 mm(C6/7). In 31–40 years group, SAC was 5.4±0.5 mm(C3/4), 4.6±0.5 mm (C4/5), 4.9±0.6 mm (C5/6), and 5.3±0.6 mm (C6/7); but among 41–50 years group, SAC was 3.8±0.6 mm (C3/4), 3.9±0.6 mm (C4/5), 3.6±0.6 mm (C5/6), and 4.3±0.6 mm (C6/7). In females SAC was 4.9±1.3 mm(C3/4), 4.5±1.2 mm(C4/5), 4.6±1.2 mm(C5/6), and 4.8±1.1 mm (C6/7). In males, SAC was 4.9±1.4 mm(C3/4), 4.6±1.2 mm(C4/5), 4.5±1.5 mm(C5/6), and 5.1±1.3 mm(C6/7). From analysis of variance, impact of age on SAC was 0.118 (p=0.001) while gender had 0.078 (p=0.223). SAC at each level has positive correlation of 0.6 to 0.7 with adjacent levels (p<0.0001). Comparing this result with studies worldwide, our population has lower SAC values than others.
Conclusion
C4/5 and C5/6 are narrowest subaxial cervical spine levels and probably explain preponderance of C4/5 and C5/6 cord injury. There may be higher incidence of congenital canal stenosis predisposing to worse outcome following cervical spine injury or degenerative diseases in this study population. This is different from European series but similar to Japanese.
The space available for the spinal cord (SAC) is the cerebrospinal fluid space around the spinal cord. It is a direct indicator for stenosis as well as a useful tool for prediction of risk of myelopathy and prognosis following spinal cord injury in an individual4,9). SAC has been argued to be a reliable canal stenosis indicator when measured with magnetic resonance imaging (MRI). Measurement with plain radiographs and even with plain computed tomography are not as reliable1,5,14).
Many factors, including race, sex, and age have been demonstrated to influence the SAC4,6,10,11). This implies that it may be wrong to extrapolate measurements derived from one study population to all populations. There is growing local burden of cervical spinal cord injury and early onset degenerative cervical myelopathy observed in the indigenous population in Nigeria. While this may partly be due to the increasing life expectancy, it is to some extent related to increasing availability of spine services in the country. There is therefore need to derive this cervical spine stenosis index for the Nigerian population.
This study analyzed the normal dimensions of the MRI derived SAC in the subaxial cervical spine of asymptomatic young adults in Enugu, Nigeria and compared the result with the literature to identify if any geographical difference exists.
This is an MRI derived prospective, cross-sectional study using asymptomatic adult Nigerians between 21 years and 50 years old that reside in Enugu who met the inclusion criteria. The MRI facility at Memfys Hospital for Neurosurgery Enugu was used. Measurements were obtained at the disc levels. The study excluded individuals that participate in any form of contact sports activities, non-Nigerians, individuals with any symptom referable to the cervical spine or spinal cord, people with congenital deformities that may suggest predisposition to congenital canal stenosis, previous cervical spine surgeries, MRI scan finding of cervical spine disease or people with contraindications to the use of MRI. A total sample size of 102 young adults was selected using multistaged sampling from among students of the University of Nigeria and individuals that presented for MRI investigation for non-spine related indications. All patients signed informed consent before participating in the study. T1-weighted MRI scans were obtained and used in this study. T2 images were however obtained to rule out any subtle cervical spine pathology. Individuals that consented for the study had MRI of the cervical spine with BASDA 0.35T MRI machine (Schenzen BASDA Medical Apparatus Co., LTD., Shenzhen, China). Midsagittal disc-level spinal canal dimension (Y) was taken as well as the corresponding level spinal cord dimension(X). SAC was calculated as Y–X(Fig. 1).
Data was analyzed using both descriptive and inferential statistics aided by the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). To assess the contributory effect of sex, age and subaxial spine levels on the value of SAC, a multivariate and univariate analysis of variance tests were conducted. This study was approved by the research ethical board of Memfys Hospital (MH/ADMIN/C.112). All the subjects signed informed consent.
Average values of SAC were 4.9±1.4 mm(C3/4), 4.5±1.2 mm (C4/5), 4.6±1.4 mm(C5/6), 4.9±1.2 mm(C6/7), and 5.6±1.5 mm at C7/T1 (Table 1). In 21–30 years group, SAC was 5.4±0.6 mm(C3/4), 4.9±0.6 mm(C4/5), 4.9±0.6 mm(C5/6), and 5.1±0.5 mm(C6/7) (Table 2). In the 31–40 years age group, SAC was 5.44±0.5 mm(C3/4), 4.6±0.5 mm(C4/5), 4.9±0.6 mm(C5/6), 5.3±0.5 mm(C6/7); but among 41–50 years group, SAC was 3.8±0.6 mm(C3/4), 3.9±0.6 mm(C4/5), 3.6±0.6 mm(C5/6), and 4.3±0.6 mm(C6/7). There was statistically significant effect of age on the SAC(p=0.0001) (Table 2). From analysis of variance (ANOVA) test, the impact of age on SAC was 0.118 (p= 0.001) (Table 3). Follow-up ANOVA indicates that the effect of age on SAC across the subaxial cervical spine levels varied from 0.316 at C3/4 (p=0.0001); 0.247 at C7/T1 (p=0.0001); 0.228 at C5/6 (p=0.0001); 0.213 at C6/7 (p=0.001); 0.145 at C4/5 (p= 0.015) (Table 4).
At C3/4 level, SAC in females was 4.9±1.3 mm and 4.9±1.4 mm in males; at C4/5 it was 4.5±1.2 mm in females but 4.6±1.2 mm in males; at C5/6 SAC measured 4.6±1.2 mm in females and 4.5±1.5 mm in males while at C6/7 SAC measured 4.8±1.1 mm in females but 5.1±1.3 mm in males. At C7/T1, SAC value was 5.5±1.4 mm in the females and 5.7±1.5 mm in the males (Table 5). There was no statistically significant difference in the values of SAC between the sex groups across different disc levels (p=0.241). The mean difference between females and males at C3/4 was 0.045 mm(p=0.870), −0.73 mm at C4/5 (p=0.770), 0.076 mm at C5/6 (p=0.782), −0.337 mm at C6/7 level (p=0.168) (Table 5). From ANOVA test, the effect of sex on SAC was 0.078 (p=0.223) (Table 3).
The correlation between the SAC at C3/4 with SAC at C4/5 was 0.686 (p=0.001), 0.650 at C5/6 (p=0.001), 0.635 at C6/7 (p=0.001), and 0.599 at C7/T1 (p=0.001). The correlation between SAC at C4/5 and C5/6 was 0.722 (p=0.001), 0.684 with C6/7 (p=0.001), and 0.612 with C7/T1 (p=0.001). The correlation between SAC at C5/6 and C6/7 was 0.750 (p=0.001) and 0.645 with C7/T1 (p=0.001). SAC at C6/7 has a correlation value of 0.676 with C7/T1 (p=0.001) (Table 6).
The finding from this study revealed that the C4/5 and C5/6 levels are the narrowest segments in the subaxial cervical spine in both sexes and across different age groups amongst adult Nigerians. Since the spinal cord attains the maximal cervical spine dimension at the same levels due to brachial plexus enlargement further compromising the SAC, this anatomical narrowing may contribute to the preponderance of cervical cord injury at these levels.
Relatively, the SAC values obtained at each subaxial cervical spine level in this study environment were much smaller when compared to the findings from America and Eastern Europe7,9,14). It is of interest however that the findings in our study were more similar to the findings among the Japanese population3) (Table 7). A similar pattern of racial difference between Caucasians and Blacks was also observed in separate studies carried out in the United States and South Africa11,12). Both genetic and environmental factors may explain the racial differences observed between this study and other parts of the world.
These findings have some significance. The relatively low SAC from this study population when compared to other studies also suggests that there may be significant racial disparity in the expected outcome of spinal cord injured patients. The findings of low SAC may also indicate an increased predisposition of the study population to congenital cervical spinal canal stenosis and the subsequent possible risk of posttraumatic and degenerative myelopathy especially around the C4/5 level. Although other factors like mechanism of injury and duration of pressure on the spinal cord are known to affect outcomes in myeloradiculopathy, individuals with small SAC or sagittal canal dimension as observed in this study population have been shown in previous studies to be at an increased risk of myelopathy as well as recurrence of neurapraxia8,9,11,13,15).
Age has significant effect on SAC and contributes as high as 11.8% effect to the variations in the values of SAC. This indicates that the linear composite of SAC differs at different age groups. This effect is most marked at C3/4 level with 31.6% impact. We also observed that among the subaxial cervical spine segments measured, the transition levels (C3/4 and C7/T1) had the highest variation in SAC values. This implies that cervical spine trauma is likely to accelerate degenerative disease at these transition levels.
Although many studies using symptomatic adults show a statistically significant difference between genders5,6,12), subgroup analysis in this study of asymptomatic adults found that the SAC did not show overall significant sex variation and gender contributed just 7% to variations in the value of SAC. Even at the different intervertebral disc levels measured, only the C6/7 and C7/T1 levels had any significant variation in the average value of SAC between males and females although females generally had slightly higher SAC at other levels. The finding in this study may be a reflection of the social demographics of the samples since the study was centered in the city. In urban areas like Enugu, sex roles are gradually becoming less defined. In addition increasingly more women are participating in contact sports activities. Therefore, with these changing patterns of social behavior, the likelihood of differential exposure of a particular gender to the risk factors that accelerate degenerative changes is likely to be low. The result may be different if the study were carried out in the rural areas where for example the men, who are mainly farmers and artisans, are expected to carry heavy loads compared with the females who also have their defined sex roles such as housekeeping, cooking, and petty trading.
Another possible reason for the absence of sex difference is the methodology of this study. This study took measurements at the intervertebral disc level unlike most of the other studies that highlighted sex differences which took measurements at the midvertebral body level. We however believe that measurement taken at the disc level may be more sensitive and predictive of the risk of myeloradiculopathy. This finding is not isolated. Wong et al.16) observed no gender difference in Pavlov ratio and midsagittal canal dimensions although the study was carried out among patients that already presented with significant clinical symptoms and without age restriction. Lee et al.5) and Hukuda and Kojima2) in separate studies also found a similar trend of no gender difference in midsagittal canal dimensions respectively.
Despite some limitations, this study has helped to establish reference values for SAC as a more direct cervical canal stenosis indicator for this study population. These values will form useful baseline for further studies and research in this field for this environment. It will also provide a base for clinical screening, early identification of individuals with pre-existing threat to the cervical spinal cord and follow-up of patients for early surgical decision making. Early identification and advice would help to reduce the risk of exposure from participation in certain high risk occupational, social and contact sports activities for individuals with increased predisposition to cervical spinal cord injury from pre-existing congenital or acquired canal stenosis especially at a relatively young age. Furthermore, the small value of SAC from this study population relative to results among Caucasians and other parts of the world implies that the risk of spinal cord injury is higher in this environment. Therefore every effort should be made to improve primary prevention strategies in order to minimize the already high morbidity and mortality associated with spinal cord injury. This requires access to health education, improved awareness of the risks of cervical spine injury, early detection of individuals at risk using reliable screening methods and frequent medical follow-up whenever indicated. Increased awareness of a pre-existing cervical canal stenosis may contribute immensely to reduction in the rate of permanent spinal cord injury for identified high risk patients.
In the study environment with a poor health seeking behaviour, significant proportion of patients still present to the expert quite late. Considering the higher possibility of onset of neurological deficit as a result of the reduced SAC in this study population compared to the Caucasians, a management model based on screening for early identification of patients at risk and subsequent clinical follow-up for future management decision may possibly be a better model than the current approach that relies solely on review of symptomatic patients. This would ensure that patients are operated on as soon as they become symptomatic before irreversible spinal cord injury occurs.
The findings from this study has strengthened the need for a local baseline of canal stenosis indicators instead of relying on data from other countries for screening, diagnosis or patient counseling since the clinical predictive value of data from other races would be poor in this study population. Further longitudinal clinical studies are needed to better clarify the usefulness of these measurements for screening, diagnosis and early surgical decision in patients at risk for myeloradiculopathy.
C4/5 and C5/6 are the narrowest subaxial cervical spine levels among asymptomatic adults with mean SAC of 4.4±1.2 mm and 4.6±1.2 mm, respectively and probably explain the preponderance of C4/5 and C5/6 cord injury. This is different from European series but similar to Japanese series. However, there is no significant gender difference in the values of SAC of asymptomatic adults. Further clinically correlated studies would help to refine the mean SAC MSCSAC measurements into a more reliable clinical tool for the screening, advice of young adults for the level of risk of developing posttraumatic and degenerative myeloradiculopathy, early diagnosis of chronic stingers and as a decision guide for timing of cervical spinal decompressive surgery.
CONFLICT OF INTEREST
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Fig. 1
T1-weighted magnetic resonance imaging of the midsagittal cervical spine with illustration of measurements obtained.
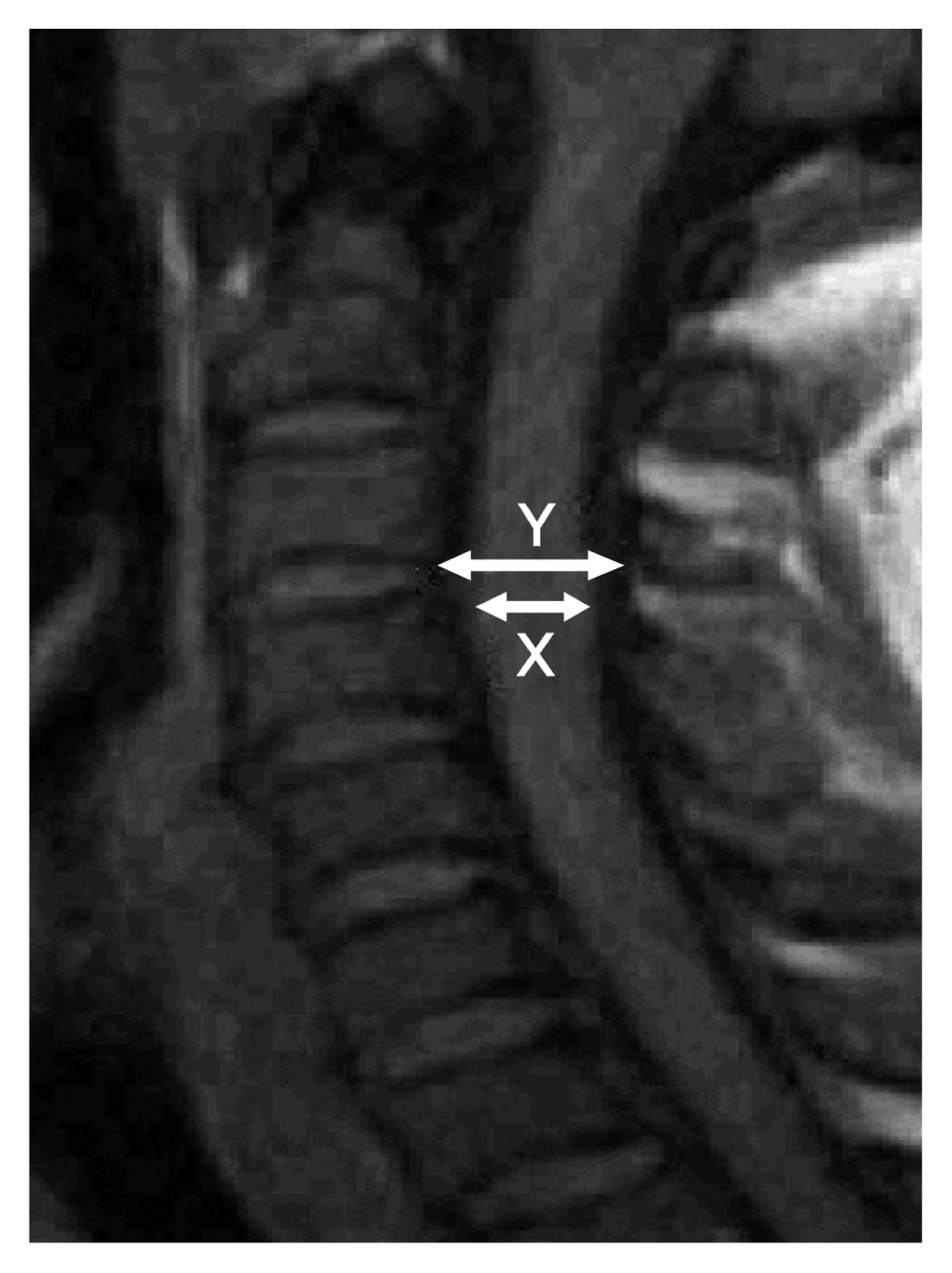
Table 1
Values of SAC at each subaxial cervical spine level
| Spine level | Mean±SD (mm) |
|---|---|
| SAC3 | 4.92±1.36 |
| SAC4 | 4.52±1.24 |
| SAC5 | 4.56±1.37 |
| SAC6 | 4.97±1.22 |
| SAC7 | 5.61±1.45 |
Table 2
Normal age adjusted range of SAC values at each subaxial spine level
Table 3
Multivariate analysis of effect of age and sex on the SAC
Table 4
Univariate analysis of variance of effect of age on all the subaxial cervical spine levels
| Age & level | F-value | p-value | Partial eta squared (effect size) |
|---|---|---|---|
| Age | |||
| SAC3 | 8.134 | 0.000 | 0.316 |
| SAC4 | 2.993 | 0.015 | 0.145 |
| SAC5 | 5.198 | 0.000 | 0.228 |
| SAC6 | 4.754 | 0.001 | 0.213 |
| SAC7 | 5.778 | 0.000 | 0.247 |
Table 5
Effect of sex on the normal values of SAC at each subaxial spine level
Table 6
Correlation between SAC at adjacent segments
| Spine level | SAC3 | SAC4 | SAC5 | SAC6 |
|---|---|---|---|---|
| SAC4 | ||||
| Pearson correlation | 0.686*** | - | - | - |
| Sig. (2-tailed) | 0.000 | - | - | - |
| SAC5 | ||||
| Pearson correlation | 0.650*** | 0.722*** | - | - |
| Sig. (2-tailed) | 0.000 | 0.000 | - | - |
| SAC6 | ||||
| Pearson correlation | 0.635*** | 0.684*** | 0.750*** | - |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | - |
| SAC7 | ||||
| Pearson correlation | 0.599*** | 0.612*** | 0.645*** | 0.676*** |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 |
Table 7
Mean SAC at different subaxial cervical spine levels from different studies
| Authors (country) | Age (yr) | Sample | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|---|---|
| Matveeva et al. 2012 (Croatia)7) | 26–64 | 30 | 7.7 | 6.9* | 7.0 | 7.7 | 8.7 |
| Prasad et al. 2003 (UK)8) | 20–40 | 87 | 43 | 4.0* | 4.5 | 5.4 | - |
| Tierney et al. 2002 (USA)14) | 24±2.5 | 14 | 5.6 | 5.3* | 5.3* | 5.7 | 7.1 |
| Presciutti et al. 2009 (USA)9) | 20–30 | 119 | 6.6 | 6.5* | 6.7 | 6.8 | - |
| Inoue et al. 1996 (Japan)3) | 13–69 | 36 | 4.4 | 4.2* | 4.5 | 4.8 | 5.6 |
| This study (Nigeria) | 20–50 | 102 | 4.9 | 4.5* | 4.6 | 4.9 | 5.6 |
REFERENCES
1. Herzog RJ, Wiens JJ, Dillingham MF, Sontag MJ. Normal cervical spine morphometry and cervical spinal stenosis in asymptomatic professional football players. Plain film radiography, multiplanar computed tomography, and magnetic resonance imaging. Spine (Phila Pa 1976) 16(6 Suppl):S178-186. 1991.


2. Hukuda S, Kojima Y. Sex discrepancy in the canal/body ratio of the cervical spine implicating the prevalence of cervical myelopathy in men. Spine (Phila Pa 1976) 27:250-253. 2002.


3. Inoue H, Ohmori K, Takatsu T, Teramoto T, Ishida Y, Suzuki K. Morphological analysis of the cervical spinal canal, dural tube and spinal cord in normal individuals using CT myelography. Neuro-radiology 38:148-151. 1996.

4. Ishikawa M, Matsumoto M, Fujimura Y, Chiba K, Toyama Y. Changes of cervical spinal cord and cervical spinal canal with age in asymptomatic subjects. Spinal Cord 41:159-163. 2003.



5. Lee HM, Kim NH, Kim HJ, Chung IH. Mid-sagittal canal diameter and vertebral body/canal ratio of the cervical spine in Koreans. Yonsei Med J 35:446-452. 1994.


6. Lim JK, Wong HK. Variation of the cervical spinal Torg ratio with gender and ethnicity. Spine J 4:396-401. 2004.


7. Matveeva N, Lazarova D, Nakeva N, Zivadinovik J, Zafirova B. Cervical spinal canal measurements: indicators of spinal stenosis. Acta Morphol 9:20-23. 2012.
8. Prasad SS, O’Malley M, Caplan M, Shackleford IM, Pydisetty RK. MRI measurements of the cervical spine and their correlation to Pavlov’s ratio. Spine (Phila Pa 1976) 28:1263-1268. 2003.


9. Presciutti SM, DeLuca P, Marchetto P, Wilsey JT, Shaffrey C, Vaccaro AR. Mean subaxial space available for the cord index as a novel method of measuring cervical spine geometry to predict the chronic stinger syndrome in American football players. J Neurosurg Spine 11:264-271. 2009.


10. Siemionow K, An H, Masuda K, Andersson G, Cs-Szabo G. The effects of age, sex, ethnicity, and spinal level on the rate of intervertebral disc degeneration: a review of 1712 intervertebral discs. Spine (Phila Pa 1976) 36:1333-1339. 2011.



11. Taitz C. Anatomical observations of the developmental and spondylotic cervical spinal canal in South African blacks and whites. Clin Anat 9:395-400. 1996.


13. Teresi LM, Lufkin RB, Reicher MA, Moffit BJ, Vinuela FV, Wilson GM, et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology 164:83-88. 1987.


14. Tierney RT, Maldjian C, Mattacola CG, Straub SJ, Sitler MR. Cervical spine stenosis measures in normal subjects. J Athl Train 37:190-193. 2002.



- TOOLS
-
METRICS

-
- 7 Crossref
- Scopus
- 7,745 View
- 154 Download
-
Journal Impact Factor 3.8
SURGERY: Q1
CLINICAL NEUROLOGY: Q1
- Related articles in NS
-
An Unusual Stab Injury of the Cervical Spinal Cord - A Case Report -.2005 June;2(2)





























