- Search
|
|
||
Abstract
Radiographic confirmation of fusion after anterior cervical discectomy and fusion (ACDF) surgery is a critical aspect of determining surgical success. However, there is a lack of established diagnostic radiographic parameters for pseudoarthrosis. The purpose of this study is to summarize the findings of previous studies, review the advantages and disadvantages of frequently employed diagnostic criteria, and present our recommended protocol of fusion assessment. This study identified randomized controlled trials, case-control studies, and prospective and retrospective cohort studies reporting on spinal fusion and how successful fusion after ACDF. Among the 39 articles reviewed, bridging bone across the operated levels on static radiographs was the most commonly used criteria to confirm fusion (31 of 39, 79%). Dynamic flexion-extension radiographs were used to assess for interspinous movement (ISM) (22 of 39, 56.4%) and change in Cobb angle (12 of 39, 30.8%). Computed tomography (CT) based findings (21 of 39, 53.8%) were employed in ambiguous cases with improved sensitivity and specificity. Reconstructed CT scans were used to assess for intragraft bridging bone and extragraft bridging bone (ExGBB). ExGBB were proved to have the highest diagnostic sensitivity and specificity for pseudoarthrosis detection when compared to all other radiographic criteria. The ISM <1 mm on dynamic flexion-extension radiographs had high diagnostic sensitivity and specificity as well. After our reviewing, we recommend using dynamic lateral flexion-extension cervical spine radiographs at 150% magnificationin which the interspinous motion <1 mm and superjacent interspinous motion ≥4 mm confirms fusion. In ambiguous cases, we recommend using reconstructed CT scans to evaluate for ExGBB.
The aim of anterior cervical discectomy and fusion (ACDF) surgery is to provide the patient with adequate decompression and rapid fusion to treat cervical degenerative disease, ultimately reducing symptoms of neck pain, radiculopathy, and myelopathy. Since its introduction in the 1950s, ACDF has a proven track record of high fusion rates [1]. However, pseudoarthrosis and adjacent segment disease are known complications of the procedure that may lead to persistent symptoms requiring further revisions surgery which is complicated by prolonged hospital stay and increased morbidity [1-4]. The true etiology of pseudoarthrosis is difficult to ascertain, but there are known risk factors, which include patient factors and surgical factors such as multilevel fusions, instrumentation choice and bone grafts used for the case [5-7]. The reported fusion rate following anterior cervical spine surgery with fixation is as high as 96% [8], but the majority of reoperation after the anterior approach was due to pseudoarthorsis [9]. Numerous imaging modalities and diagnostic radiographic criteria to determine the fusion status following ACDF have been described and used in recent literature. However, only a few of these radiographic parameters were rigorously analyzed to validate their accuracy and reliability so far. This inconsistency in reporting pseudoarthrosis following ACDF stems from a lack of established radiographic parameters in the literature. This current study aims to review recent literature over the last decade (2008 to 2018) to evaluate the different imaging modalities and radiographic parameters used in prior works. In this review article, we will summarize the findings of previous studies, review the advantages and disadvantages of frequently employed diagnostic criteria, and present our recommended protocol of fusion status assessment with a case example.
This study identified longitudinal studies including randomized controlled trials (RCT), observational case-control studies, and prospective and retrospective cohort studies reporting on the success of spinal fusion and how successful fusion was identified and defined after ACDF. The National Institutes of Health PubMed database was queried using a combination of free and medical subject headings search parameters related to the surgical intervention (e.g., “anterior cervical discectomy and fusion,” “ACDF,” “cervical arthrodesis”) and outcome of interest (e.g., “fusion,” “pseudoarthrosis,” “nonunion,” “treatment outcomes”) in major journals. There were no language restrictions on potential studies. Studies that were published within the approximately 10-year period between January 1, 2008 and June 30, 2018 were included for inclusion in this study to assess for recent trends in radiographic pseudoarthrosis diagnosis. Studies without clear radiographic fusion criteria, literature review, case reports, and fusion assessment of upper cervical spine and craniocervical junction were excluded. A list of relevant articles was identified using these search terms by 3 authors (WL, AH, VB) and were manually screened for inclusion in this current review. All longitudinal studies that reported on a cohort of patients who underwent ACDF for any indication and who were followed postoperatively for fusion status were included in this study.
After an initial screen of abstracts and article titles, we obtained full text articles of all potential studies. Three reviewers independently assessed the articles using the inclusion criteria until a consensus was reached. Relevant data identified from each article was the type of study (RCT, case-control, cohort studies), level of evidence, number of patients included in the study, number of intervertebral levels assessed in the study, imaging modality used to assess spinal fusion (e.g., plain films, computed topography [CT], etc.), and how fusion was assessed from imaging (e.g., trabecular bone bridge, motion, etc.).
In total, 39 articles (Table 1) published between January 1, 2008 and June 30, 2018 met our inclusion criteria [10-49]. The various imaging modalities used to assess fusion status in these studies included radiographs (static and dynamic lateral) and CT imaging. No studies were identified that reported cervical spine fusion or pseudoarthrosis based solely on history or physical examination.
The most frequently used radiographic diagnostic criteria for pseudoarthrosis after ACDF was an absence of bridging trabeculae across the fused levels on static radiographs (31 of 39 used this definition, 79%) [10-40]. Fusion status was also frequently assessed using dynamic lateral radiographs. Using this imaging technique, authors measured ISM (22 of 39, 56.4%) [10,13,14,16,19-22,25,28,29,31,33-39,41-43] or used the Cobb angle method (12 of 39, 30.8%) [11,15,17,27,32,34,37,42-46] to assess cervical fusion. No consensus was reached regarding the amount of motion for evaluation of cervical fusion on dynamic lateral radiographs. For the interspinous process method, no motion (9 of 22, 40.9%) [13,14,17,21,24,26,29,33,37], under 1 millimeter (mm) (7 of 22, 31.8%) [10,20,28,31,32,41,42], under 2 mm (2 of 22, 9.1%) [12,35], and under 3 mm of motion (4 of 22, 18.2%) [27,30,40,43] between spinous process were all used as cutoff values for a definition of fusion. Using the Cobb angle method, changes of 1 degree (1 of 12, 8.3%) [38], 1.5 degrees (1 of 12, 8.3%) [42], 2 degrees (5 of 12, 41.7%) [19,25,36,44,46], 4 degrees (4 of 12, 25.0%) [11,43,45], and 5 degrees (2 of 12, 16.7%) [27,30] were all used in various studies.
CT scans were the most commonly used advanced imaging modality to assess fusion status (21/39, 53.8%) [10,12,15,16,19,22,26,29,33-38,41,43,45-49]. Fusion was identified in these studies by assessing for continuous bridging bone at the cage or graft endplate interface. Pseudoarthrosis was identified as a radiolucent gap across the fused levels. Reconstructed multi-axial CT scans were also compiled to assess for intragraft bone bridging (InGBB) and extragraft bone bridging (ExGBB) [18,32].
Achieving fusion after ACDF is critical to attain predictable postoperative pain relief and functional recovery. Pseudoarthrosis is an uncommon, but known complication after ACDF that leads to persistent unresolved symptoms, which often requires revision surgeries [1-4]. Although the gold standard to assess pseudoarthrosis is operative exploration of fusion mass, the preoperative radiographic options to determine fusion status is still poorly described in the literature. The pseudoarthrosis rate is affected by patient factors (diabetes, smoking, etc.), fusion levels, graft choice, and surgical instrumentation, but the true etiology remains difficult to establish given high rates of asymptomatic patients and inconsistent diagnostic radiographic parameters [32,50-53]. Therefore, the purpose of this review study was to inspect the articles published over the past decade to assess which imaging modality and radiographic parameters were used to diagnose fusion after ACDF to identify current trends and consensus protocols for radiographical assessment of fusion status.
In this systematic review study of 39 articles published between January 1, 2008 and June 30, 2018, the fusion status after ACDF was confirmed with different imaging modalities in addition to the history and physical exam of the patients. All the studies included cervical spine radiographs as one of the tools used to assess the fusion status and most studies used more than one criterion, which suggests a more stringent criteria for fusion assessment since our previous review article [53]. The use of cervical spine films as a first line to diagnose pseduoarthrosis stems from low costs, easy accessibility, and low radiation for the patient.
Static anteroposterior and lateral cervical spine films are consistently described as the initial approach to assess ACDF postoperative fusion status in recent literature. These films expose the patients to low-dose radiation and provide the clinician with valuable information regarding fusion status. In the static films, fusion is confirmed with formation of osseous bridging between the graft and vertebral body across the fused levels. Pseudoarthrosis is diagnosed with static films when there is an absence of this osseous bridging and a presence of haloing or lucency around the graft [10,33]. However, pseudoarthrosis diagnosed in plain films only correlates between 43%–82% with pseudoarthrosis detected during operative exploration of fusion mass, which is most likely secondary to the inability of films to accurately assess bony morphology with an implant in place [54]. Among the 39 included articles, only two studies determined cervical spine fusion status or pseudoarthrosis based solely on static radiographs as the only modality [23,40]. Given the lower rate of pseudoarthrosis detection using just the static films, the recent trends for pseudoarthorsis diagnosis have moved from relying on static radiographs to dynamic radiographs and advanced imaging. The dynamic lateral flexion-extension cervical spine films provide an improved method of assessing postoperative ACDF fusion status. The dynamic films can either evaluate for the ISM or the change in Cobb angle, both of which have a wide range of diagnostic radiographic parameters in literature. The ISM measurement was much more prevalent in recent works, which accounted for more than half of the literature reviewed in this study. The ISM measurement leading to no movement (40.9%) [13,14,17,21,24,26,29,33,37] and the Cobb angle change of less than 2 degrees (41.7%) [19,25,36,44,46] were the most common method to confirm fusion after ACDF in recent literature. Song et al. [20] evaluated the accuracy of different radiographic parameters used to assess fusion status after ACDF and reported that less than 1 mm of ISM showed the best accuracy and agreement with intraoperative exploration. In this study, he demonstrated increased inter and intraobserver reliability of ISM measurement using magnified 150% radiographs compared to the 25% and 100% magnification, which was observed in future studies as well [20,31]. Another comparative study conducted by Riew et al. [32] demonstrated that <1 mm ISM cutoff value showed a reliable accuracy compared with conventional CT based bridging bone criteria and showed acceptable sensitivity and specificity second only to ExGBB. Although no motion in ISM is frequently used in recent literature to validate fusion, there is concern that this method may increase the number of reported pseudoarthrosis since micromotion still exists within the solidly fused levels on dynamic films. On the other hand, the cutoff value of less than 2 mm of ISM measurement may overestimate fusion rate, leading to missed diagnosis of pseudoarthrosis. Also, there is no literature confirming the validity of no motion or less than 2 mm of ISM as a reliable method of pseudoarthrosis detection. An important aspect of measuring the ISM is to also be mindful of the superjacent ISM which should be more than 4 mm to increase negative predictive value and sensitivity. Although the no motion of ISM is frequently described in recent literature as a method of pseudoarthrosis diagnosis, only the ISM less than 1mm have been shown to have reliable accuracy.
The dynamic flexion-extension films can also assess for the change in Cobb angle between the adjacent fused vertebrae to determine postoperative fusion status. Recent literature frequently employed the change in Cobb angle as a criteria to confirm fusion (30.8%) [11,15,17,27,32,34,37,42-46], but the radiographic parameters ranged from 1.5 to 5 degrees. Cannada et al. [4] showed that changes in Cobb angle of 2 degrees lead to a sensitivity of 82% and specificity of 39%. This significantly improved with the Cobb angle change of 4 degrees that resulted in a specificity of 100% [4]. However, the Cobb angle measurement may be a less reproducible form of radiographic parameter compared to the ISM measurement. The Cobb angle measurement is closely associated with the instantaneous center of rotation while obtaining the dynamic cervical spine films, making an accurate and consistent angle measurement difficult. Although there is evidence that combining quantitative motion analysis software with dynamic radiographs may yield objective and reliable numbers compared to manual or subjective measurements, the limitation of specialized technology and software availability make this less useful [42,47]. The difficulty of consistently reproducing the Cobb angle measurement makes this a less appealing method to diagnose pseudoarthrosis for the authors.
In addition to plain radiographs, CT scans were frequently used (53.8%) [10,12,15,16,19,22,26,29,33-38,41,43,45-49], to assess pseudoarthrosis following ambiguous radiographic findings. The interobserver reliability of predicting pseudoarthrosis is better using the CT scan despite the metal artifacts compared to dynamic cervical spine films [10]. The radiographic parameter for CT scan based fusion diagnosis is still in flux in recent literature. Kim et al defined bony fusion as “fused with remodeling and trabeculae present” or “graft intact, not fully remodeled and incorporated, but no lucency present,” which is more of a general vague description of fusion status [48]. Other CT scan based parameters used the lack of motion in fused segments and at times no specific parameter was described for fusion assessment [14,28,29,37,47,55]. Song et al. [18] first described the ExGBB and InGBB on CT scans to subcategorize the areas of achieved fusion in 2013. Riew et al. [32] further evaluated the ExGBB and InGBB in multiaxial reconstructed CT scans and reported that ExGBB had the highest sensitivity and specificity and acceptable accuracy to detect pseudoarthrosis, but InGBB was worse than guessing. Although the CT scan is superior to standard films in evaluating bony fusion status, it is limited to findings derived from a static moment in time. It fails to assess for dynamic changes in the cervical spine during motion, which may leave out some cases of pseudoarthrosis seen only with movement. Also, the CT scan based fusion status is based on subjective interpretation compared to the objective measurement findings on standard films, which make the results more vulnerable to both type I and type II errors [47]. In addition, because of the imaging features of cortical allografts, the CT evaluation may actually omit the nonunion and overstate the fusion rates, particularly during the early post-operative follow-up period. The published CT based pseudoarthrosis diagnosis is inconsistent with other radiographic nonunion indicators including internal fixation failure, peri-instrument halo signs, and cystic changes around the grafts [20]. Overall, the CT scan is a valuable tool used to confirm equivocal findings on standard radiographs given its improved pseudoarthrosis detection rate, but care must be taken prior to obtaining the imaging given its limitations.
Diagnosing pseduoarthrosis after ACDF is controversial and the literature is inconsistent in objectively evaluating postoperative radiographic findings. After reviewing the recent studies, we find that no one single method is absolutely perfect for the diagnosis of pseudoarthrosis. After taking the advantages and disadvantages into consideration, we recommend first using the dynamic lateral flexion-extension cervical spine films in 150% magnification as the initial method for evaluation, since this is economically prudent, quick, and most informative of dynamic cervical spine movements with low radiation for the patient. The interspinous motion <1 mm and superjacent interspinous motion ≥4 mm confirms the fusion diagnosis in the dynamic films (Fig. 1). In ambiguous cases (Fig. 2), we recommend using the reconstructed multiaxial CT scans to evaluate for ExGBB given its superior diagnostic qualities (Fig. 3).
Fig. 1.
Measurement of interspinous movement (ISM) at superjacent level (C4–5) and operated levels (C5–7) on the 150% magnified flexion and extension radiographs. The superjacent ISM at C4–5 (A and a) is 10.2 mm, which indicates adequate dynamic motion ( > 4 mm). ISM at C5–6 (B and b) is 0.21 mm, which is consistent with our definition of fusion ( < 1 mm). ISM at C6–7 (C and c) is 5.13 mm, which indicates pseudoarthrosis ( > 1 mm).
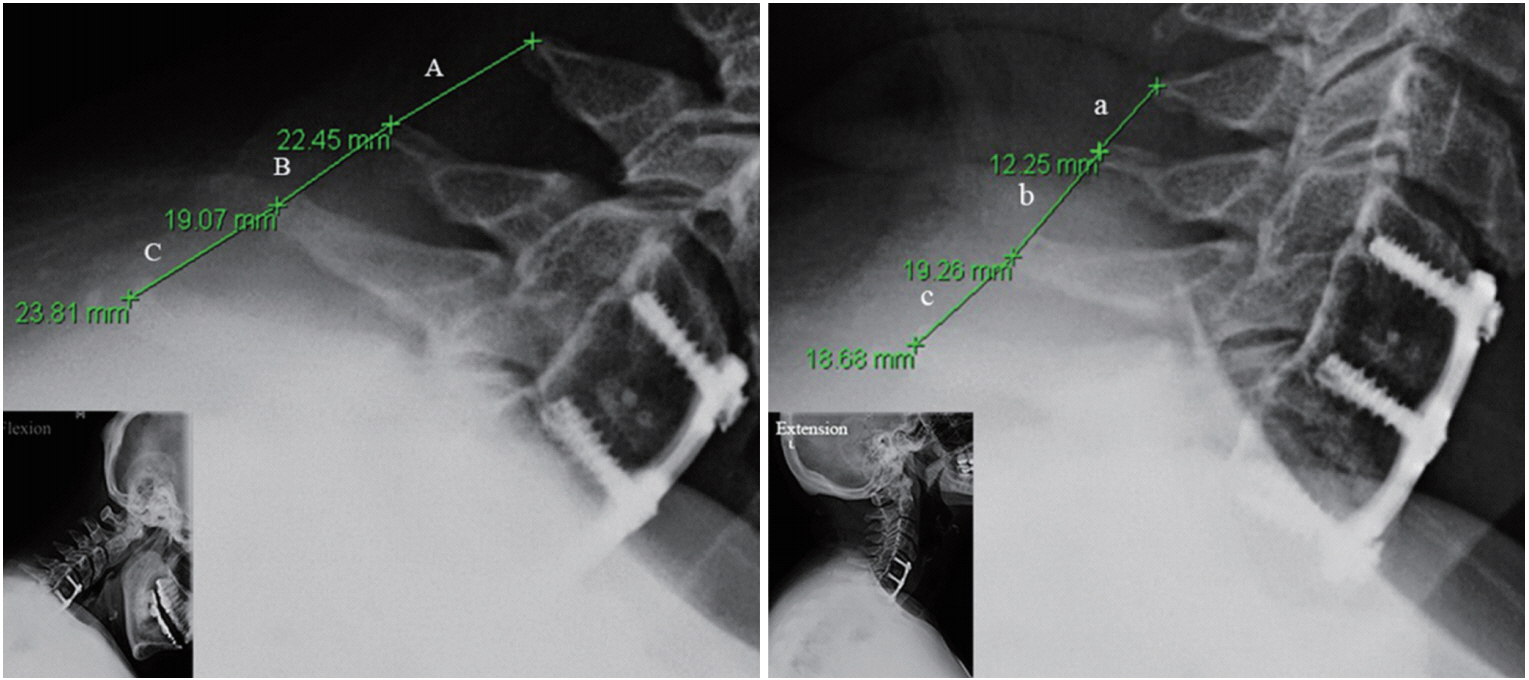
Fig. 2.
Evaluating bone bridging on multiaxial reconstructed coronal and sagittal computed tomographic images. It appears to be fused with bone bridging formation at both C5–6 (A and a) and C6–7 (B and b) levels.

Fig. 3.
Evaluating extragraft bridging bone (ExGBB) on multiaxial reconstructed coronal and sagittal computed tomographic images. The top level (C5–6) shows ExGBB on both the coronal and sagittal images (A and a). The bottom level (C6–7) shows intragraft bone bridging on the coronal view (B) but demonstrates a cleft and no ExGBB on sagittal image (b), which indicates pseudoarthrosis. This is consistent with the results of interspinous movement evaluation on X-rays and was confirmed to be pseudoarthrosis with intraoperative exploration.
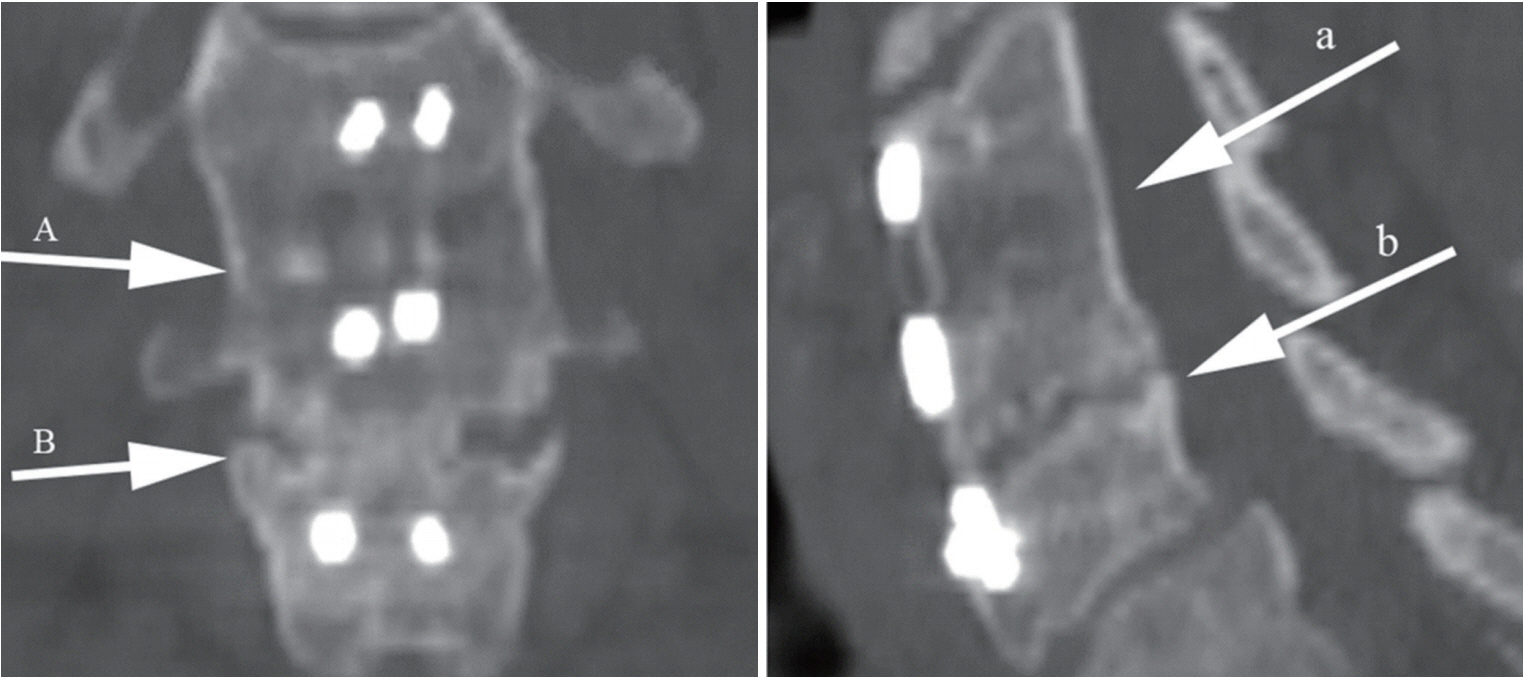
Table 1.
Literature review of methods and criteria used for determining fusion from 2008 to 2018 study
| No. | First author | Journal | Year | Type of study | Level of evidence | No. of patients | No. of intervertebral levels | Fusion assessment |
|---|---|---|---|---|---|---|---|---|
| 1 | Buchowski [3] | Spine | 2008 | Prospective clinical trial | II | 14 | N/A | R (static AP, lateral flexion/extension): bridging trabeculae visualized, <1 mm interspinous process motion on lateral flexion/extension |
| CT: bridging trabeculae seen, no bony lucency seen at graft/vertebral body junction | ||||||||
| MRI: bony bridging from endplate to endplate without signal alteration at the graft/vertebral body junction | ||||||||
| Surgical exploration | ||||||||
| 2 | Buttermann [41] | Spine J | 2008 | Prospective but nonrandomized study | II | 66 | 124 | R (lateral flexion/extension): <1 mm of gapping of the spinous process on lateral flexion/extension films |
| CT: used to assess fusion but did not specify fusion criteria on CT | ||||||||
| 3 | Chiang [22] | Spine | 2008 | Retrospective study | II | 56 | 85 | R (lateral flexion/extension): trabecular bone across the interfaces without lucencies between the cage and vertebral endplates |
| CT: bony bridging formation between superior and inferior endplates | ||||||||
| 4 | Fassett [42] | J Neurosurg Spine | 2008 | Cohort | II | 100 | 512 | R (lateral flexion/extension): distance between the spinous processes in flexion and extension was measured manually (<1 mm considered fused), and by a proprietary Qualitative Motion Software (<1.5 degrees of angular motion considered fused) |
| 5 | Foley [5] | Spine J | 2008 | A randomized, controlled, prospective multicenter clinical trial | I | 323 | N/A | R (AP, lateral flexion/extension): greater than or equal to 50% bony bridging through both surfaces of the graft-vertebra interface, no radiolucency at any portion of the graft-vertebra junction, ≤4 degrees of motion between adjacent fused vertebrae |
| 6 | Oh [33] | Spine | 2009 | Retrospective study | II | 31 | 62 | R (lateral flexion/extension): absence of motion between spinous process on flexion/extension radiographs, absence of any dark halo around iliac bone graft cages, presence of bridging bone anterior or posterior to a cage or iliac bone graft |
| 7 | Song [36] | Spine | 2009 | Retrospective study | II | 78 | N/A | R (AP, lateral flexion/extension): less than 2 degrees of movement on lateral flexion/extension, presence of bridging trabecular bone between endplates on AP/lateral views, lack of signs of implant failure of the anterior plate system, less than 50% radiolucency in the perimeter surrounding the cage |
| CT: used to assess fusion but did not specify fusion criteria on CT | ||||||||
| 8 | Tomasino [37] | J Neurosurg Spine | 2009 | Retrospective study | II | 30 | 30 | R (AP, lateral flexion/extension): absence of movement during dynamic measurements of spinous process distance on flexion-extension lateral radiographs, presence of bridging bone at the surgical level |
| CT: presence of bridging bone at the surgical level | ||||||||
| 9 | Uribe [35] | Eur Spine J | 2009 | Retrospective comparative study | II | 80 | N/A | R (AP, lateral flexion/extension): absence of motion more than 2 mm between spinous process on flexionextension lateral radiographs, absence of radiolucent gap between the graft and end plate, presence of continuous bridging trabeculae at the graft and end plate junction |
| 10 | Lofgren [38] | Eur Spine J | 2010 | Prospective randomized study | I | 80 | N/A | R (AP, lateral flexion/extension): presence of bone-bridging or interface lucencies between trabecular material and bone, measuring the differences between the angles of the spinal process of the fused vertebrae at flexion and extension (<1 degree was “clearly fused”) |
| MRI: Used to assess decompression but was not used to determine fusion status | ||||||||
| 11 | Ghiselli [47] | Spine | 2011 | Prospective study | II | 10 | 24 | R (lateral flexion/extension): distance between spinous processes in flexion and extension measured using Qualitative Motion Software |
| CT: presence of bony trabeculae across the fusion level, lack of bony lucency at the graft/vertebral body junction | ||||||||
| Surgical exploration | ||||||||
| 12 | Guo [12] | Eur Spine J | 2011 | Retrospective clinical study | II | 120 | N/A | R (lateral flexion/ extension): absence of motion > 2 mm between spinous processes on flexion-extension lateral radiographs, absence ofradiolucent gap between the graft and end plate, presence of continuous bridging trabeculae at the graft and end plate junction |
| 13 | Lebl [39] | Spine J | 2011 | Retrospective comparative cohort study | II | 29 | 29 | R (lateral flexion/ extension): presence of bony bridging across the interbody space |
| 14 | Sugawara [40] | spine | 2011 | Retrospective study | II | 105 | 165 | R (lateral flexion/ extension): motion of spinous process on flexion-extension radiographs < 3 mm, visible bony bridging between vertebral bodies, absence of halo around the cages |
| CT: sagittal reconstruction images to identify bony bridging | ||||||||
| 15 | Lin [14] | Eur Spine J | 2012 | Retrospective study | II | 120 | N/A | R (AP, lateral flexion/extension): no motion across fusion site on flexion/extension radiographs, trabeculae across the fusion site, no lucency across fusion site or around any screw sites |
| CT: sagittal reconstructive images to identify bony bridging | ||||||||
| 16 | Song [15] | Spine | 2012 | Retrospective clinical study | II | 78 | 122 | R (AP, lateral flexion/extension): presence of bridging bone between grafted bone and vertebral bodies, absence of radiolucent defects, Halo sign, or loss of grafted bone, and new bone formation in the exterior portion of cages and a partial or complete loss of radiopaque line at the endplates by sclerotic changes on the bony bridges between the vertebral endplate and grafted bone in the interior portion of the cage |
| 17 | Song [13] | Eur Spine J | 2012 | Retrospective study | II | 40 | 117 | R (AP, lateral flexion/extension): absence of motion between spinous process on flexion/ extension radiographs, absence of any radiolucent defects or Halo sign around the iliac bone graft or cages, presence of a bridging bone anterior or posterior to a cage or iliac bone graft at the graft-endplate junction |
| 18 | Barbagallo [16] | Eur Spine J | 2013 | Prospective cohort study | II | 32 | 77 | R (AP, lateral flexion/extension): no radiolucencies in the graft-endplate area and bridging trabeculae |
| CT: obtained to confirm neural decompression, rule out fracture but not to assess fusion | ||||||||
| MRI: obtained to document neural decompression but not to assess fusion | ||||||||
| 19 | Chen [17] | Eur Spine J | 2013 | Prospective,randomized, control study | II | 80 | 240 | R (lateral flexion/ extension): absence of motion between spinous processes, absence of a radiolucent gap between the graft and endplates, presence of continuous bridging bony trabeculae at the graft-endplate interface |
| 20 | Coric [19] | J Neurosurg Spine | 2013 | Prospective randomized study | II | 74 | N/A | R (AP, lateral flexion/extension): > 50% trabecular bridging bone, < 2 degrees of motion or less, and no implant loosening |
| 21 | Kim [48] | Neurosurgery | 2013 | prospective randomized study | I | 52 | N/A | R (AP, lateral flexion/extension): did not specify fusion criteria on plain films |
| CT: bony fusion defined as fused with ^modeling and trabeculae prcsent, graft intact, not fully remodeled and incorporated but with no radiolucencies present | ||||||||
| 22 | Song [18] | Spine | 2013 | Retrospective study developing diagnostic criteria | II | 110 | 254 | CT: extragraft bone bridging (ExGBB) and intragraft bone bridging (InGBB). ExGBB was defined as complete cortical bridging at any peripheral margins of the operated disc space outside of the graft, and InGBB was defined as cortical or trabecular bridging within the confines of the graft only |
| Surgical exploration | ||||||||
| 23 | Song [20] | J Bone Joint Surg Am | 2014 | Retrospective study | II | 125 | 262 | R (lateral flexion/extension): interspinous motion < 1 mm, with superjacent interspinous motion < 4 mm on 150% magnified dynamic lateral radiographs |
| Surgical exploration | ||||||||
| 24 | Grasso [23] | Eur Spine J | 2015 | Prospective comparative study | II | 20 | N/A | R (AP, lateral flexion/ extension): absence of segmental mobility, absence of radiolucencies, absence of bone sclerosis, and evidence of bridging trabecular bone within the fusion arca |
| 25 | Lau [24] | J Neurosurg Spine | 2015 | Retrospective study | II | 55 | 145 | R (AP, lateral flexion/extension): Absence ofradiolucent lines, bridging trabecular bone across the fusion site, no motion between spinous processes, no motion between vertebral bodies |
| 26 | Phillips [25] | Spine | 2015 | Prospective, randomized controlled Trial | II | 293 | 293 | R (AP, lateral flexion/ extension): evidence of continuous bridging bone between the adjacent endplates of the involved motion segment, radiolucent lines at < 50% of the graft-vertebra interfaces, < 2 degrees of segmental rotation |
| 27 | Shi [46] | Spine J | 2015 | Retrospective comparative study | II | 38 | 114 | R (lateral flexion/extension): < 2 degrees of motion on flexion/ extension radiographs, absence of a radiolucent gap between the graft and the endplate. |
| 28 | Wang [21] | Eur Spine J | 2015 | Retrospective clinical study | II | 63 | N/A | R (lateral flexion/extension): absence of motion between the spinous processes on dynamic lateral radiographs, absence of a radiolucent gap between the graft and endplates, presence of continuous bridging bony trabeculae at the graft endplate interface |
| CT: used to assess fusion but did not specify fusion criteria on CT | ||||||||
| 29 | Arnold [27] | Spine | 2016 | Prospective, randomized, controlled, trial | II | 319 | N/A | R (AP, lateral flexion/ extension): evidence of bridging trabecular bone between the involved motion segments, translational motion < 3 mm and angular motion < 5 degrees |
| CT: trabecular bone formation patterns within the intervertebral disc space or bridging bone formation that crossed the interspace | ||||||||
| 30 | Chen [44] | Eur Spine J | 2016 | Retrospective study | II | 54 | 162 | R (lateral flexion/extension): < 2 degrees of motion on flexion/ extension radiographs, no radiolucent gap between graft and endplate |
| CT: no radiolucent gap between graft and endplate | ||||||||
| 31 | Lki [26] | Eur Spine J | 2016 | Retrospective clinical study | II | 60 | N/A | R (AP, lateral flexion/ extension): absence of motion between spinous processes, absence ofradiolucent gap between graft and endplate, presence of continuous bridging boy trabeculae at the graft-endplate interface |
| CT: absence of motion between spinous processes, absence ofradiolucent gap between graft and endplate, presence of continuous bridging boy trabeculae at the graft-endplate interface | ||||||||
| 32 | Vanichkachorn [45] | Eur Spine J | 2016 | Prospective clinical study | II | 31 | N/A | R (lateral flexion/extension): < 4 degrees of angular motion |
| CT: bridging bone across the adjacent endplates on thin cut CT scans with sagittal and coronal reconstructions | ||||||||
| 33 | Li [29] | Eur Spine J | 2017 | Retrospective clinical study | II | 152 | N/A | R (AP, lateral flexion/extension): no motion across the fusion site, trabeculae across the fusion site, no radiolucencies across fusion or screw sites |
| CT: obtained to assess fusion status but did not specify fusion criteria on CT | ||||||||
| MRI: obtained to assess adjacent segment degenerative changes, not to assess fusion status | ||||||||
| 34 | Arnold [30] | Neurosurgery | 2018 | Prospective, randomized, controlled, multicenter clinical trial | I | 319 | N/A | R (AP, lateral flexion/ extension): evidence of bridging trabecular bone between the involved motion segments and translational motion < 3 mm and angular motion < 5 degrees |
| CT: trabecular bone formation patterns within the intervertebral disc space or bridging bone formation that crossed the interspace | ||||||||
| 35 | Basques [34] | Eur Spine J | 2018 | Retrospective cohort study | II | 404 | N/A | R (AP, lateral): anterior and posterior bone bridging was present |
| 36 | Buttermann [28] | Spine | 2018 | Prospective cohort study | II | 159 | N/A | R (lateral flexion/extension): continuous trabeculation and no > 1 mm splaying of the tips of the spinous processes of the fused level |
| CT: used to assess fusion but did not specify fusion criteria on CT | ||||||||
| 37 | Feng [43] | Eur Spine J | 2018 | Prospective randomized controlled study | II | 55 | 90 | R (AP, lateral flexion/extension): rotation < 4 degrees and < 1.25 mm translation with the absence of motion adjacent to interspinous processes (〉3 mm) in the flexion/extension view |
| CT: prcsence of continuous trabecular bone bridging either anterior, posterior, or within the PEEK cage | ||||||||
| 38 | Lee [31] | Spine | 2018 | Retrospective comparative study | III | 89 | 151 | R (lateral flexion/extension): interspinous distance change of < 1 mm on > 150% magnification, presence of bridging bone across the graft into the adjacent endplates and/ or bridging bone outside the graft, radiolucent lines extending < 50% from the cortical-host bone interface |
| CT: presence of bridging bone across the graft into the adjacent endplates and/ or bridging bone outside the graft, radiolucent lines extending < 50% from the cortical-host bone interface | ||||||||
| 39 | Riew [32] | Spine J | 2018 | Retrospective radiographic comparative study | II | 82 | 151 | R (lateral flexion/extension): interspinous movement < 1 mm at the arthrodesis level and interspinous movement > 4 mm at a non-arthrodesed superjacent level based on 150% magnified dynamic radiographs |
| CT: presence of bridging bone and/ or the lack of radiolucency at the graft-vertebral junction, presence of ExGBB and InGBB Surgical exploration |
REFERENCES
1. Fraser JF, Hartl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine 2007 6:298-303.


2. Aronson N, Filtzer DL, Bagan M. Anterior cervical fusion by the smith-robinson approach. J Neurosurg 1968 29:396-404.


3. Cloward RB. Vertebral body fusion for ruptured cervical discs. Description of instruments and operative technic. Am J Surg 1959 98:722-7.


4. Cannada LK, Scherping SC, Yoo JU, et al. Pseudoarthrosis of the cervical spine: a comparison of radiographic diagnostic measures. Spine (Phila Pa 1976) 2003 28:46-51.


5. Jagannathan J, Shaffrey CI, Oskouian RJ, et al. Radiographic and clinical outcomes following single-level anterior cervical discectomy and allograft fusion without plate placement or cervical collar. J Neurosurg Spine 2008 8:420-8.


6. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine (Phila Pa 1976) 1991 16(10 Suppl):S561-4.


7. Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 1993 75:1298-307.


8. Kaiser MG, Haid RW Jr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery 2002 50:229-36.


9. Zhu B, Xu Y, Liu X, et al. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J 2013 22:1583-93.



10. Buchowski JM, Liu G, Bunmaprasert T, et al. Anterior cervical fusion assessment: surgical exploration versus radiographic evaluation. Spine (Phila Pa 1976) 2008 33:1185-91.


11. Foley KT, Mroz TE, Arnold PM, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J 2008 8:436-42.


12. Guo Q, Bi X, Ni B, et al. Outcomes of three anterior decompression and fusion techniques in the treatment of three-level cervical spondylosis. Eur Spine J 2011 20:1539-44.



13. Song KJ, Lee KB, Song JH. Efficacy of multilevel anterior cervical discectomy and fusion versus corpectomy and fusion for multilevel cervical spondylotic myelopathy: a minimum 5-year follow-up study. Eur Spine J 2012 21:1551-7.



14. Lin Q, Zhou X, Wang X, et al. A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J 2012 21:474-81.


15. Song J, Taghavi CE, Hsu DW, et al. Radiological changes in anterior cervical discectomy and fusion with cage and plate construct: the significance of the anterior spur formation sign. Spine (Phila Pa 1976) 2012 37:272-9.


16. Barbagallo GM, Romano D, Certo F, et al. A single institution series with four years maximum follow-up and review of the literature on zero-profile devices. Eur Spine J 2013 22 Suppl 6:S868-78.

17. Chen Y, Wang X, Lu X, et al. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7-year follow-up. Eur Spine J 2013 22:1539-46.



18. Song KS, Chaiwat P, Kim HJ, et al. Anterior cervical fusion assessment using reconstructed computed tomographic scans: surgical confirmation of 254 segments. Spine (Phila Pa 1976) 2013 38:2171-7.


19. Coric D, Kim PK, Clemente JD, et al. Prospective randomized study of cervical arthroplasty and anterior cervical discectomy and fusion with long-term follow-up: results in 74 patients from a single site. J Neurosurg Spine 2013 18:36-42.


20. Song KS, Piyaskulkaew C, Chuntarapas T, et al. Dynamic radiographic criteria for detecting pseudarthrosis following anterior cervical arthrodesis. J Bone Joint Surg Am 2014 96:557-63.


21. Wang Z, Jiang W, Li X, et al. The application of zero-profile anchored spacer in anterior cervical discectomy and fusion. Eur Spine J 2015 24:148-54.


22. Chiang CJ, Kuo YJ, Chiang YF, et al. Anterior cervical fusion using a polyetheretherketone cage containing a bovine xenograftp: three to five-year follow-up. Spine (Phila Pa 1976) 2008 33:2524-428.


23. Grasso G. Clinical and radiological features of hybrid surgery in multilevel cervical degenerative disc disease. Eur Spine J 2015 24 Suppl 7:842-8.



24. Lau D, Chou D, Mummaneni PV. Two-level corpectomy versus three-level discectomy for cervical spondylotic myelopathy: a comparison of perioperative, radiographic, and clinical outcomes. J Neurosurg Spine 2015 23:280-9.


25. Phillips FM, Geisler FH, Gilder KM, et al. Long-term outcomes of the US FDA IDE prospective, randomized controlled clinical trial comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2015 40:674-83.


26. Liu Y, Wang H, Li X, et al. Comparison of a zero-profile anchored spacer (ROI-C) and the polyetheretherketone (PEEK) cages with an anterior plate in anterior cervical discectomy and fusion for multilevel cervical spondylotic myelopathy. Eur Spine J 2016 25:1881-90.



27. Arnold PM, Sasso RC, Janssen ME, et al. Efficacy of i-Factor Bone Graft versus Autograft in anterior cervical discectomy and fusion: results of the prospective, randomized, singleblinded food and drug administration investigational device exemption study. Spine (Phila Pa 1976) 2016 41:1075-83.


28. Buttermann GR. Anterior cervical discectomy and fusion outcomes over 10 years: a prospective study. Spine (Phila Pa 1976) 2018 43:207-14.


29. Li Z, Zhao Y, Tang J, et al. A comparison of a new zero-profile, stand-alone Fidji cervical cage and anterior cervical plate for single and multilevel ACDF: a minimum 2-year followup study. Eur Spine J 2017 26:1129-39.



30. Arnold PM, Sasso RC, Janssen ME. i-FactorTM Bone Graft vs Autograft in anterior cervical discectomy and fusion: 2-year follow-up of the randomized single-blinded food and drug administration investigational device exemption study. Neurosurgery 2018 83:377-84.



31. Lee DH, Cho JH, Hwang CJ, et al. What is the fate of pseudarthrosis detected 1 year after anterior cervical discectomy and fusion? Spine (Phila Pa 1976) 2018 43:E23-8.


32. Riew KD, Yang JJ, Chang DG, et al. What is the most accurate radiographic criterion to determine anterior cervical fusion? Spine J 2018 Jul 7 [Epub]. pii: S1529-9430(18)30648-X. https://doi.org/10.1016/j.spinee.2018.07.003.

33. Oh MC, Zhang HY, Park JY, et al. Two-level anterior cervical discectomy versus one-level corpectomy in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2009 34:692-6.


34. Basques BA, Louie PK, Mormol J, et al. Multi- versus singlelevel anterior cervical discectomy and fusion: comparing sagittal alignment, early adjacent segment degeneration, and clinical outcomes. Eur Spine J 2018 Jun 26 [Epub]. htps://doi.org/10.1007/s00586-018-5677-y.


35. Uribe JS, Sangala JR, Duckworth EA, et al. Comparison between anterior cervical discectomy fusion and cervical corpectomy fusion using titanium cages for reconstruction: analysis of outcome and long-term follow-up. Eur Spine J 2009 18:654-62.



36. Song KJ, Taghavi CE, Lee KB, et al. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine (Phila Pa 1976) 2009 34:2886-92.


37. Tomasino A, Gebhard H, Parikh K, et al. Bioabsorbable instrumentation for single-level cervical degenerative disc disease: a radiological and clinical outcome study. J Neurosurg Spine 2009 11:529-37.


38. Lofgren H1, Engquist M, Hoffmann P, et al. Clinical and radiological evaluation of Trabecular Metal and the Smith-Robinson technique in anterior cervical fusion for degenerative disease: a prospective, randomized, controlled study with 2-year follow-up. Eur Spine J 2010 19:464-73.


39. Lebl DR, Bono CM, Metkar US, et al. Bioabsorbable anterior cervical plate fixation for single-level degenerative disorders: early clinical and radiographic experience. Spine J 2011 11:1002-8.


40. Sugawara T, Itoh Y, Hirano Y, et al. β-Tricalcium phosphate promotes bony fusion after anterior cervical discectomy and fusion using titanium cages. Spine (Phila Pa 1976) 2011 36:E1509-14.


41. Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J 2008 8:426-35.


42. Fassett DR, Apfelbaum RI, Hipp JA. Comparison of fusion assessment techniques: computer-assisted versus manual measurements. J Neurosurg Spine 2008 8:544-7.


43. Feng SW, Chang MC, Chou PH, et al. Implantation of an empty polyetheretherketone cage in anterior cervical discectomy and fusion: a prospective randomised controlled study with 2 years follow-up. Eur Spine J 2018 27:1358-64.



44. Chen Y, Lu G, Wang B, et al. A comparison of anterior cervical discectomy and fusion (ACDF) using self-locking standalone polyetheretherketone (PEEK) cage with ACDF using cage and plate in the treatment of three-level cervical degenerative spondylopathy: a retrospective study with 2-year follow-up. Eur Spine J 2016 25:2255-62.



45. Vanichkachorn J, Peppers T, Bullard D, et al. A prospective clinical and radiographic 12-month outcome study of patients undergoing single-level anterior cervical discectomy and fusion for symptomatic cervical degenerative disc disease utilizing a novel viable allogeneic, cancellous, bone matrix (trinity evolutionTM) with a comparison to historical controls. Eur Spine J 2016 25:2233-8.



46. Shi S, Liu ZD, Li XF, et al. Comparison of plate-cage construct and stand-alone anchored spacer in the surgical treatment of three-level cervical spondylotic myelopathy: a preliminary clinical study. Spine J 2015 15:1973-80.


47. Ghiselli G, Wharton N, Hipp JA, et al. Prospective analysis of imaging prediction of pseudarthrosis after anterior cervical discectomy and fusion: computed tomography versus flexion-extension motion analysis with intraoperative correlation. Spine (Phila Pa 1976) 2011 36:463-8.


48. Kim CH, Chung CK, Hahn S. Autologous iliac bone graft with anterior plating is advantageous over the stand-alone cage for segmental lordosis in single-level cervical disc disease. Neurosurgery 2013 72:257-65.



49. Leven D, Cho SK. Pseudarthrosis of the cervical spine: risk factors, diagnosis and management. Asian Spine J 2016 10:776-86.



50. Bolesta MJ, Rechtine GR 2nd, Chrin AM. One- and twolevel anterior cervical discectomy and fusion: the effect of plate fixation. Spine J 2002 2:197-203.


51. Hilibrand AS, Fye MA, Emery SE, et al. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg Am 2001 83-A:668-73.

52. Lau D, Chou D, Ziewacz JE, et al. The effects of smoking on perioperative outcomes and pseudarthrosis following anterior cervical corpectomy: clinical article. J Neurosurg Spine 2014 21:547-58.


53. Sethi N, Devney J, Steiner HL, et al. Diagnosing cervical fusion: a comprehensive literature review. Asian Spine J 2008 2:127-43.




- TOOLS






























