- Search
|
|
||
Abstract
Neuromuscular disorders (NMDs) are diseases involving the upper and lower motor neurons and muscles. In patients with NMDs, cervical spinal deformities are a very common issue; however, unlike thoracolumbar spinal deformities, few studies have investigated these disorders. The patients with NMDs have irregular spinal curvature caused by poor balance and poor coordination of their head, neck, and trunk. Particularly, cervical deformity occurs at younger age, and is known to show more rigid and severe curvature at high cervical levels. Muscular physiologic dynamic characteristics such as spasticity or dystonia combined with static structural factors such as curvature flexibility can result in deformity and often lead to traumatic spinal cord injury. In addition, postoperative complication rate is higher due to abnormal involuntary movement and muscle tone. Therefore, it is important to control abnormal involuntary movement perioperatively along with strong instrumentation for correction of deformity. Various methods such as botulinum toxin injection, physical therapy, muscle division technique, or intrathecal baclofen pump implant may help control abnormal involuntary movements and improve spinal stability. Surgical management for cervical deformities associated with NMDs requires a multidisciplinary effort and a customized strategy.
Neuromuscular disorders (NMDs) are a heterogeneous collection of various diseases and diagnoses caused by abnormalities in upper motor neurons, lower motor neurons, peripheral nerves, neuromuscular junctions, and muscles. NMDs frequently involve several organ systems and show various clinical manifestations; for example, Duchenne muscular dystrophy involves skeletal muscle, smooth muscle, myocardial, endocrine, brain, and ocular abnormalities, which cause a variety of symptoms [1]. The overall prevalence of NMDs is approximately 72.6/100,000 population, ranging from <0.2/100,000 population to 24.9/100,000 population for each disease (e.g., cerebral palsy, 9.8/100,000 population; multiple sclerosis, 18.8/100,000 population) in previous studies. Most NMDs have a prevalence lower than 50/100,000 population, making them very rare diseases [2,3].
From the perspective of spine surgeons, spinal deformities which are one of the most challenging problems of the spine, are well known to be disproportionately common in patients with NMDs [4]. In a previous report, spinal deformities were present in 20% to 70% of cerebral palsy patients, depending on the severity of the disease, with prevalence rates of 60% in patients with Friedreich ataxia, 80% in patients with spinal muscular atrophy (SMA), and 85% to 90% in male patients with Duchenne muscular dystrophy [4-6]. Since the prevalence of spinal deformities requiring treatment in the adolescent population without NMDs is approximately 4 to 7 cases per 1,000, the relative prevalence of spinal deformities in patients with NMDs is clearly much higher [4].
Occipito-cervical or cervical area is a common region with spinal problems in patients with NMDs. Since the cervical spine has a wide range of motion and complex functions, involuntary movement in NMDs can cause the development of an excessive range of motion, resulting in cord compression that requires surgical treatment. These factors can generate a wide range of disorders and alignment pathologies necessitating surgical treatment. In addition to structural parameters such as thoracic kyphosis, other indicators reflecting the severity of NMD such as the Hoehn and Yahr stage are also associated with cervical positive sagittal malalignment [7]. However, although cervical spine deformities and NMDs have unique characteristics and substantially impact patients’ lives, few comprehensive studies have focused on cervical deformities in patients with NMDs. Therefore, in this article, we reviewed the existing literature on cervical spinal deformity surgery and sought to identify relevant surgical strategies in patients with NMDs.
Although the characteristics or types of spinal deformities in NMDs may be similar in each disease, the natural history or nuances of each underlying NMD that could affect spinal curvature may be different. The progression of degenerative changes throughout the whole spine in patients with NMDs is more severe than in patients without NMDs. Spinal deformity is a sequela of childhood-onset NMDs and some adults with NMDs. The prevalence of scoliosis varied from 33% to 100% in the literature. By understanding the natural history of spinal deformities, we might be able to predict the probability and pattern of curve progression, and on that basis develop a treatment plan (e.g., the decision between surgical or conservative treatment), obtain informed consent from patients, and educate patients appropriately. However, most existing studies have mainly focused on the natural history of thoracolumbar curvature, and few studies have investigated cervical curvature [8-10]. In addition, as NMDs themselves are rare, there is a tendency for reports to focus on cerebral palsy, which is a relatively common NMD [11]. Generally, it has been reported that deformity curve progression is more common in patients with NMDs than in adolescent idiopathic scoliosis (AIS) patients with similar patterns [12]. Also, unlike AIS, deformities in patients with NMDs occur more frequently along with curve progression, even in adulthood after growth, so NMD patients need constant follow-up in adulthood. The course of NMDs itself affects deformity curve progression. For example, progressive NMDs such as muscular dystrophy are associated with more predictable patterns of deformity progression than nonprogressive NMDs such as cerebral palsy [4]. In a limited number of studies regarding curve progression in untreated neuromuscular scoliosis, most cases of neuromuscular scoliosis were found to occur before 10 years of age. The amplitude of the curve, whether the curve is thoracolumbar or lumbar, bedridden status, underlying disease severity, mental retardation, and functional status are factors known to be related to curve progression. Gu et al. [13] reported that patients with a Cobb angle > 40° by the age of 12 were more likely to experience progression than those with a Cobb angle of ≤ 40° by the same age among 110 pediatric patients with a diagnosis of spastic tetraplegic cerebral palsy. Thometz and Simon [14] reported that curve progression was 0.8° each year in patients with curves less than 50°, whereas it was 1.4° in those with a curve more than 50°; they also reported that progression was more common in patients with spastic quadriplegia, a thoracolumbar or lumbar curve, and bedridden status. Some studies have also investigated spinal deformities in patients with Duchenne muscular dystrophy and pediatric spinal cord injuries, reporting that both conditions are associated with a high incidence of spinal deformity and risk of progression [15,16].
Apart from the curve progression of the spinal deformity, the progression of NMDs is known to cause pain and alterations in skin integrity, difficulty in ambulation and position maintenance, and most seriously, pulmonary and cardiac compromise, ultimately causing mechanical effects on thoracic volume and compliance. Galasko et al. [17] found that deterioration of pulmonary function occurred simultaneously with rapid spinal deformity progression in untreated Duchenne muscular dystrophy patients.
Cervical diseases in people with NMDs have different characteristics compared to those in people without NMDs. Cervical deformities in NMD patients are usually more rigid and have more severe curve and dystonic muscle characteristics. Furthermore, cervical deformities in patients with NMDs exhibit different characteristics from those in non-NMD patients with existing cervical spondylotic myelopathy (CSM). According to a study by Nishihara et al. [18], CSM tended to occur at a younger age in cerebral palsy patients, whereas it was otherwise most common in patients in their 50s. Additionally, the most frequently affected sites tended to be at higher levels (e.g., C3–4 and C4–5) [18,19]. According to White and Panjabi et al. [20], C5–6 has the largest flexion and extension in the subaxial cervical spine, and lateral bending and axial rotation are greater at C3–4 and C4–5 than at C5–6. However, in cerebral palsy patients, lateral bending and axial rotation were estimated to be greater than flexion and extension compared to normal adults. Therefore, C3–4 and C4–5 are most common sites of spondylotic changes in cerebral palsy patients. In addition, in these cerebral palsy patients, adult spinal deformity is more likely to recur with significant stress and strain in the adjacent intervertebral disc of the fused vertebrae.
In cervical and thoracolumbar deformities, several factors might be able to cause spinal asymmetries, such as asymmetric paraplegia, altered strength and muscle tone, intraspinal and congenital anomalies, impaired sensory feedback, abnormal spinal balance, and pelvic obliquity. Furthermore, abnormal movement, which is one of the major characteristics of NMDs, is well known to cause spinal deformities. Generally, abnormal movement in NMDs refers to neurological syndromes that include excessive and involuntary or nonspontaneous and nonautomatic movements that occur regardless of weakness or spasticity. Some of these abnormal movements are caused by damage to brain nuclei such as the basal ganglia. Increasing or reducing the inhibitory output activity of abnormal basal ganglia reportedly causes movement disorders. Depending on the increased inhibitory activity of the motor cortical or brain stem area, paucity of movement appears, and hyperkinesia such as chorea or ballism is caused by abnormal neuronal signaling from the basal ganglia to the thalamus or cortex [21]. Consequently, abnormalities in the neuromuscular system result in dysfunction of the muscles that keep the spine in a balanced posture, which causes the spine to become deformed. Muscle dysfunction or weakening has a significant effect on spine deformities [22,23]. In the report of Yagi et al. [24], the multifidus and iliopsoas muscles were reported to be small in deformity patients, which were also correlated with sagittal alignment. In addition, Kim et al. [22] and Shafaq et al. [23] reported different muscle patterns on the convex side and the concave side of scoliosis. Due to these characteristics, the development of neuromuscular spinal deformities tends to progress even after bone growth is completed because the imbalance of the muscles that support the spine lasts for a lifetime [14,25,26].
Meanwhile, the Research Group on Extrapyramidal Disorders categorized these abnormal movements into 2 major groups, the first group being disorders of movement and the second group being disorders of posture and tone [27,28]. Disorders of movement include hypokinesia and hyperkinesia, as mentioned above, while the latter category includes hypertonia and hypotonia, dystonia, torsion spasm, the cogwheel phenomenon, and rigidity. The characteristics and classification of these abnormal movements should be considered when establishing surgical strategies for spine surgeons. In patients with hyperkinesia and hypertonia, controlling involuntary movements or increased muscle tone is the key to the success of surgery by improving stability and maintaining a balanced position. Hypokinesia and hypotonia require strong instrumentation to fully support the inactive agonist muscles. To date, few studies have reported on the pathogenesis of cervical deformities. Based on these muscular physiological characteristics in abnormal movement patterns, cervical deformities in patients with NMDs could be broadly classified into hyperkinetic and hypokinetic cervical deformities. Hyperkinetic diseases may include cerebral palsy, dystonia, and Parkinson disease, and hypokinetic diseases may include hypertonic cerebral palsy or dystonia, hypotonic amyotrophic lateral sclerosis (ALS), SMA, and myopathy. Depending on muscle tone, hypokinetic conditions could be classified into hypokinetic hypertonic types, such as cerebral palsy and dystonia, and hypokinetic hypotonic types such as ALS, myopathy, and SMA (Fig. 1).
Although there is no separate classification for cervical deformities in patients with NMDs, the spinal scoliosis classification published by the Scoliosis Research Society (SRS) may also be applied to cervical deformities. In the SRS classification of NMDs, neuropathy and myopathy are broadly divided according to the anatomical location of the causative disease, and neuropathy is further divided into upper neuropathy and lower neuropathy (Table 1) [4].
Understanding perioperative concerns unique to cervical deformities associated with NMDs can optimize postoperative care, such as pain control, patient satisfaction, etc. An appropriate history and physical examination must be obtained, as well as an understanding of the patient’s expectations from the surgery. In addition, the multidisciplinary assistance such as cardiologists, pulmonologist, or pediatrician to ensure the patient is medically optimized for surgery is very helpful. In their institutional experience with appropriate patient selection and postoperative management aided by excellent anesthesiologists, the average length of time intubated postoperatively and the average stay of intensive care unit are able to sufficiently reduce between 2 and 3 days. On preoperative radiographic work-up, anteroposterior and lateral scoliosis films are obtained along with traction and bending films, which assist in determining flexibility of the curve and the appropriate levels for instrumentation. The need for combined anteroposterior surgery is required to achieve adequate correction. Preoperative 3-dimensional computed tomography scans may be used specifically to ascertain the bony anatomy for placement of spinal instrumentation. The use of preoperative magnetic resonance imaging (MRI) is reserved for those cases whereby spinal cord abnormalities are suspected.
The surgical indications for cervical deformities are a progressive ongoing deformity with worsening of the horizontal gaze, severe pain, dysphagia, skin breakdown, and worsening of cardiac or pulmonary compromise [29,30]. Recently, as anesthesiologic techniques have developed, it has become possible to actively extend surgical indications for cervical deformities to encompass supportive care in patients with NMDs. Therefore, the purpose of cervical deformity surgery in patients with NMDs is to achieve solid fusion, maintaining a balanced position in both the coronal and sagittal planes and, eventually, maintaining a horizontal line of gaze and position of the head over the pelvis, as in surgery for other degenerative or idiopathic deformities. Surgery also improves swallowing and respiration and prevents neurological deterioration. However, most importantly, the purpose of surgical treatment is not to cure fundamentally the underlying disease; instead, the ultimate goal of surgery in these patients is to help them live independently and to restore function as much as possible.
Neurological conditions requiring surgery, such as cervical myelopathy or radiculopathy, can be caused by either static mechanical factors (bone and ligament) or dynamic factors (muscle and neuron). While static mechanical factors cause neural compression by stenosis of the spinal canal or neural foramen from excessive spondylotic changes, dynamic factors involve repetitive compression due to spine instability caused by sustained involuntary movements in NMDs [31,32]. In other words, static anatomical factors result in problems with stenosis caused by bone or ligament, and dynamic factors correspond to problems of incoordination of the nerve or muscle. Excessive involuntary movement in NMDs itself not only causes early degenerative changes (degenerative disc, herniated lumbar intervertebral disc, osteophytes), but also spinal instability [33,34]. As described above, whatever the underlying disease is, the imbalance in static mechanical and dynamic factors due to biomechanical changes of the neuromuscular structure is thought to be one of the main causes of neuromuscular spinal deformity. Therefore, controlling these abnormal movements in NMDs is an important principle of surgery. Even after successful surgery, cervical deformity in NMDs is associated with a higher incidence of postoperative complications such as pseudoarthrosis or instrument failure due to involuntary movement. Therefore, it is also important to control these involuntary movements postoperatively. In particular, if dystonic movement is reduced even for several months after index surgery, it may be possible to increase the likelihood of achieving fusion. Methods to help control abnormal involuntary movements and to improve spinal stability in patients with NMDs include botulinum toxin injection, the muscle division technique, or intrathecal baclofen pump implantation.
Botulinum toxin binds to cholinergic nerve endings of the neuromuscular junction, thereby reducing the release of acetylcholine, blocking neuromuscular transmission, and reducing muscular overactivity. Thus, botulinum toxin injection has been suggested as a treatment of choice for cervical dystonia [35-37]. According to a Cochrane review, botulinum toxin injection in cervical dystonia led to a reduction of 8.16 points on the Toronto Western Spasmodic Torticollis Rating Scale compared to the placebo group [38]. The successful use of botulinum toxin to treat cerebral palsy has also been reported [39,40]. Nonetheless, variable effects of botulinum toxin injection in cervical deformity patients with NMDs have been reported to date. The effects of perioperative botulinum toxin remain unclear, and further research is needed.
Muscle division aims to reduce involuntary movement by directly destroying overactive muscles. In patients with existing congenital muscular torticollis, the effect of this procedure remains controversial. However, in a review written by Kim et al. [41], it was reported that the success rate of surgical release was 81%. In another study, Kim et al. [42] reported significant improvement in radiological parameters such as the cervicomandibular angle and Cobb angle in 87 cervicothoracic scoliosis patients with congenital muscular torticollis. Side effects include cosmetic changes associated with permanent somatosensory loss or dysesthesia, scarring, or muscle atrophy [43].
An intrathecal baclofen pump might be another treatment option. Baclofen, an agonist of the gamma-aminobutyric acid type B receptor, blocks the release of this excitatory neurotransmitter by interfering with voltage-gated calcium channels [44]. This reduces muscle tone and prevents reflexive muscle contraction. Currently, this technique is used at many centers to treat spasticity and secondary generalized dystonia of cerebral or spinal origin. However, there is a high rate of complications such as infection, cerebrospinal fluid leak, and instrument failure (e.g., catheter or pump malfunction), and a study reported that the rate of total adverse events was 37% [45,46].
Moreover, in cervical deformities, kyphosis in the sagittal plane is more common and problematic than scoliosis or angulation in the coronal plane [47]. It is also important when planning deformity surgery in cervical kyphosis to consider whether the deformity is rigid or fixed and the location of the apex of cervical kyphosis (C0–2 or C3–T1). In the craniocervical junction (C0–1–2) deformity, craniocervical junctional osteotomy is indicated when the deformity is irreducible and sufficient to result in severe pain, functional impairment, or neurological impairment that cannot be relieved with a surgical decompression and/or stabilization procedure alone.
When apex of cervical spine deformity is localized at subaxial spine (C3–T1), surgeons could choose one of several surgical options according to curve flexibility (flexible vs. rigid) as well as location of apex of kyphosis (C3–C6 vs. C7–T1). In cases of a flexible deformity without neurological symptoms, posterior stabilization (C2–T3) is advocated; when neurological symptoms are present, an additional posterior decompression should be considered. If deformity is not fully flexible, additional minimal facetectomy or Smith Peterson type osteotomy is indicated. However, in the clinical setting of rigid cervical kyphosis at the midcervical spine with neurological symptoms, the spinal cord is usually tethered over the subaxial kyphotic segment (C3–6), leading to neurological symptoms and myelopathy. Therefore, in the mid cervical spine rigid kyphosis, segmental kyphosis correction using circumferential osteotomy is required to untether and decompress the spinal cord. In the setting of rigid cervical kyphosis in the low subaxial cervical spine, a C7 or T1 pedicle subtraction osteotomy may be sufficient (Fig. 2).
Based on the physiological characteristics of muscles with abnormal movement patterns and curve flexibility, as discussed above, we can develop a customized surgical plan.
In hyperkinetic cervical deformities, excessive spondylotic changes and severe dynamic instability can occur as a result of excessive involuntary unusual movements, and these sustained problems eventually lead to myeloradiculopathy and malalignment of the cervical spine. Therefore, the focus of surgical treatment should be placed on decompression of the neural element and strong mechanical fixation to prevent pseudoarthrosis or instrument failure. Control of abnormal involuntary neck movements, such as through botulinum toxin injection, is critically important for achieving a successful surgical outcome. In addition, correction of the deformity is also very important. Kim et al. [39] reported that botulinum toxin injection significantly lowered the incidence of a second operation in a 5-year follow-up study of 24 athetoid cerebral palsy patients. In addition, the kyphotic group of patients with cerebral palsy had a higher rate of non-favorable outcomes. According to Lee et al. [48], Neck Disability Index improvement was higher in the cervical deformity correction group than in the noncorrection group, and in terms of functional outcomes, hand grip power improved more in the correction group. Therefore, neural decompression along with correction of cervical deformity is the ideal strategy for providing better long-term surgical outcomes in hyperkinetic NMD patients with CSM.
A 50-year-old man presented with progressive weakness of both upper and lower extremities that had started several years ago. He had a history of C3-4-5 cervical anterior discectomy and fusion more than 10 years ago. On physical examination, the patient complained of both shoulder and arm pain, and both Hoffman signs were positive. Clinically, the patient showed involuntary movement of the neck preoperatively and had a dystonic posture (Supplementary video clip 1). Preoperative x-rays showed severe cervicothoracic kyphoscoliosis (Fig. 3A), and MRI demonstrated signal change of the cord and a stenotic lesion. We first performed C5-6-7 anterior cervical discectomy and fusion with a stand-alone implant and demineralized bone matrix anteriorly [49], and at the same time, the sternal head of the sternocleidomastoid muscle was bilaterally divided (Supplementary video clip 1). Then, 7 days later, C2-3-4-5-6-7-T1-2-3-4-5 fusion was performed posteriorly (Fig. 3B). There was no change on intraoperative monitoring (IOM) during surgery. Botulinum toxin injection was performed for 4 weeks after surgery.
Patients with hypokinetic hypertonic deformities show little movement, but abnormally increased muscle tone. This causes neck pain and prevents horizontal gaze maintenance. When considering surgical treatment, it is necessary to first check for the presence of other underlying neurological disorders, and then to support the cervical spine with strong circumferential fusion. As in hyperkinetic patients, it is also necessary to control the activity of hypertonic muscles through techniques such as botulinum toxin injection, muscle division, or intrathecal baclofen pump implantation.
A 30-year-old woman presented with chin on chest deformity 1 year previously. She had a history of intellectual disability and epilepsy. Her posterior neck pain increasingly deteriorated even after conservative treatment including cervical brace application and pain block, and the patient had difficulty in ambulation and maintaining a horizontal gaze. However, there was no weakness or paresthesia on physical examination. Clinically, the patient showed hypokinetic movement of the neck preoperatively and had a dystonic posture (Supplementary video clip 2). The preoperative chin brow vertical angle was -57.0° and the cervical Cobb angle was -67.3° (Fig. 4A). First, we performed anterior cervical discectomy and fusion on C3-4-5-6-7 with cutting of the bilateral sternal tendons of the sternocleidomastoid muscle. In the second stage, C2-3-4-5-6-7-T1-2-3-4 fusion was performed posteriorly with facetectomy at C3-4-5-6-7 (Fig. 4B). No changes were found on IOM during surgery. A perioperative botulinum toxin injection was also performed to control hypertonic muscle activity.
One of hypokinetic hypotonic types is dropped head syndrome (DHS). This refers to the chin on chest deformity in cervicothoracic spine due to severe weakness of the cervical paraspinal muscle (extensors), which is sometimes, unlike other fixed cervical deformity, correctable with passive neck extension. DHS is known to be caused by weakness of the posterior cervical muscle or involuntary contraction of the anterior cervical muscle accompanied by various diseases (Table 2) [50,51]. There is sometimes an isolated neck extensor myopathy that causes weakness of the cervical extensor muscle regardless of underlying neuromuscular diseases or inflammatory myopathy [52].
When establishing a treatment plan, it is necessary to understand the underlying pathology such as neuromuscular disease or autoimmune disease. Proper medical management of underlying conditions should be preceded and then surgical treatment is considered for patients who failed in nonsurgical treatment. In the absence of underlying disease, such as isolated neck extensor myopathy, steroid injection or bracing and strengthening exercise has been proposed as the first line treatment. In the case of malignant course primary neurologic disease which is not responding to these conservative treatments, the benefits of surgical treatment and conservative treatment should be weighed. If not, nonsurgical treatment should be considered the first-line management, and then if it fails, surgical treatment will be the second line management option. Even in this case, surgeons should consider whether correction of the deformity is necessary or not according to the patient’s general health status or neck disability (Fig. 5) [50,52,53].
A 62-year-old woman presented with progressive quadriparesis and intractable neck pain. She had a history of cervical tumor resection surgery 29 years ago. On physical examination, both arms and both legs had a manual muscle testing grade of 3. Clinically, the patient showed hypokinetic hypotonic movement of the neck preoperatively, with a dystonic posture (Supplementary video clip 3). We performed anterior cervical discectomy and fusion at C3-4-5-6 with an allobone graft and autologous bone. Then, on the same day, C2-3-4-5-6-7-T1-2 fusion was performed posteriorly with a autologous iliac bone graft (Fig. 6A, B). No changes were found on IOM during surgery.
As with the natural history of cervical deformities, there have been few reports of postoperative complications limited to cervical deformities in NMD patients. According to Smith et al. [54], 123 adult cervical spinal deformity patients had a 9.2% mortality rate at a mean of 1.2 year following surgery. In a review of 14 retrospective studies, Etame et al. [55] reported mortality rates ranging from 3.1% to 6.7% and major medical complication rates ranging from 3.1% to 44.4% in 399 cervical deformity patients. Generally, thoracolumbar deformity surgery in patients with NMDs is more likely to have higher complication rates than that in non-NMD patients [56-58]. Rumalla et al. [57] reported an overall complication rate of 40.1% in patients undergoing fusion surgery out of 2,154 neuromuscular scoliosis patients between 2002 and 2011. In a review study of the Scoliosis Research Society Morbidity and Mortality database, Reames et al. [59] reported that complications occurred in 1,971 cases out of a total of 19,360 cases (10.2%), including 6.3% in patients with idiopathic scoliosis, 10.6% in patients with congenital scoliosis, and 17.9% in patients with neuromuscular scoliosis, which was the highest complication rate. In addition, mortality was also reported to be higher in patients with neuromuscular scoliosis and congenital scoliosis (0.3% each). Despite variations depending on the patient or surgical parameters, the complication rate in NMD patients is also expected to be higher than that in non-NMD patients.
Owing to preexisting underlying diseases, as well as difficulties in the operation itself, patients with NMDs have more complications. According to Toll et al. [56], a 27% complication rate was reported among 102 patients, and nonambulatory status, pulmonary comorbidity, and history of seizure were the risk factors for complications. In addition, it was reported that a diagnosis of myelomeningocele and higher intraoperative blood loss were associated with a high risk of complications. Respiratory disorders like pneumonitis and pulmonary distress causing reintubation or prolonged intubation due to respiratory muscle problems are frequently observed.
Nutritional imbalances are also very common in patients with NMDs, which can lead to increased rates of postoperative complications infections. Moreover, cardiomyopathy, thromboembolic disease, gastroesophageal reflux, ileus, bladder dysfunction, nerve injury with worsening of preoperative neurological status, endocrine problems, and death also occur frequently. Therefore, it is very important to provide preoperative nutritional support and to control underlying diseases in patients with NMDs [60].
The rates of complications related to surgical procedures are also high (wound infection, 6% to 15%; fixation failure, 15%; and pseudarthrosis, 7% to 28%). These are much higher frequencies than have been reported in patients with AIS. Increased blood loss during surgery due to lengthy dissection for extensive fusion is also a very important issue in patients with NMDs. Meanwhile, from 2004 to 2015, Cognetti et al. [58] reported that there were 1,385 complications (6%) in 29,019 patients in the Scoliosis Research Society Morbidity and Mortality Database. These authors reported that there was a 3.5-fold decrease in complications during the study period, and wound infections, respiratory complications, and implant-related complications were noticeably reduced.
Neuromuscular causes of cervical deformities should be treated differently from static nonneuromuscular cervical deformities. Hyperkinetic cervical deformities have a high risk of postoperative pseudoarthrosis, neurologic deterioration, hardware failure, and progression of kyphosis. Therefore, when selecting the surgical treatment method for NMD, it is critically important to select an appropriate treatment method according to physiological muscle characteristics (e.g., whether the patient has a hyperkinetic, hypokinetic, hypertonic, or hypotonic pattern of abnormal movement). Perioperative botulinum toxin injection helps to control hyperkinetic neck motion. Dystonic muscle division is required to control intractable hyperkinesia. The understanding of pathophysiologic characteristics of cervical deformities associated with NMDs is serving to improve surgical management and decisions. If a surgical treatment for a cervical deformity with NMDs is preferred, a strong circumferential instrumentation is mandatory.
SUPPLEMENTARY MATERIALS
Supplementary video clips 1 and 2 can be found via https://doi.org/10.14245/ns.2040464.232.v1, https://doi.org/10.14245/ns.2040464.232.v2, and https://doi.org/10.14245/ns.2040464.232.v3.
Supplementary video clip 1.
Clinical video of the patient showing hyperkinetic involuntary movement and surgical procedure of muscle division.
Fig. 1.
Etiology of cervical deformity. ALS, amyotrophic lateral sclerosis; SMA, spinal muscular atrophy.
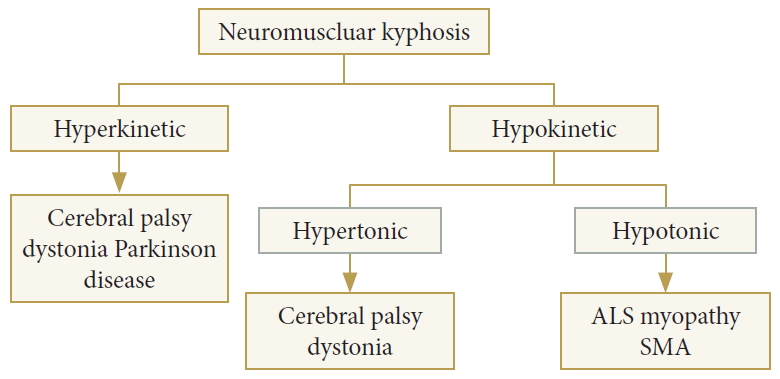
Fig. 2.
The surgical decision-making process in cervical deformity osteotomy. PSO, pedicle subtraction osteotomy.

Fig. 3.
(A) Preoperative x-rays in the hyperkinetic patient. (B) Postoperative x-rays in the hyperkinetic patient.
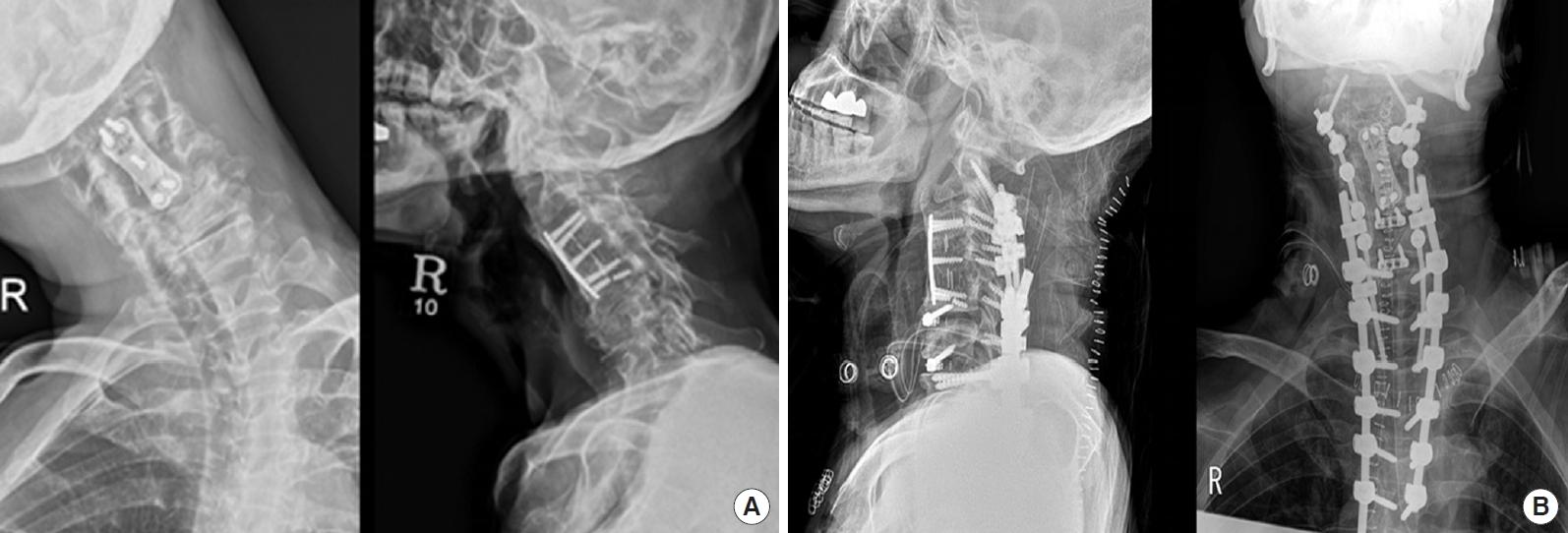
Fig. 4.
(A) Preoperative x-rays in the hypokinetic hypertonic patient with drop head symptom. (B) Postoperative x-rays in the hypokinetic hypertonic patient with drop head symptom.
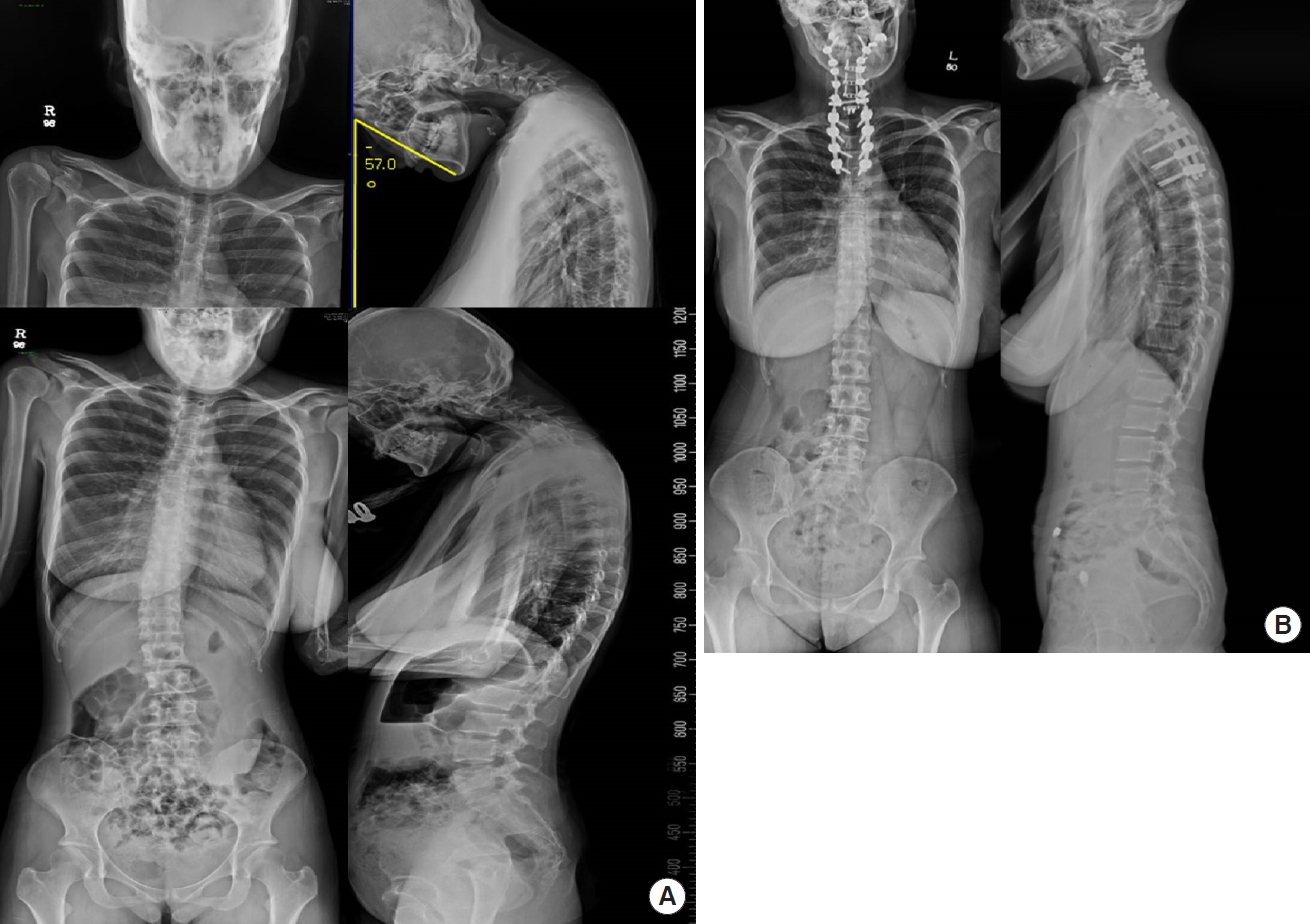
Fig. 5.
The treatment algorithm in dropped head syndrome. ext., extensor; seg., segment; NDI, Neck Disability Index.
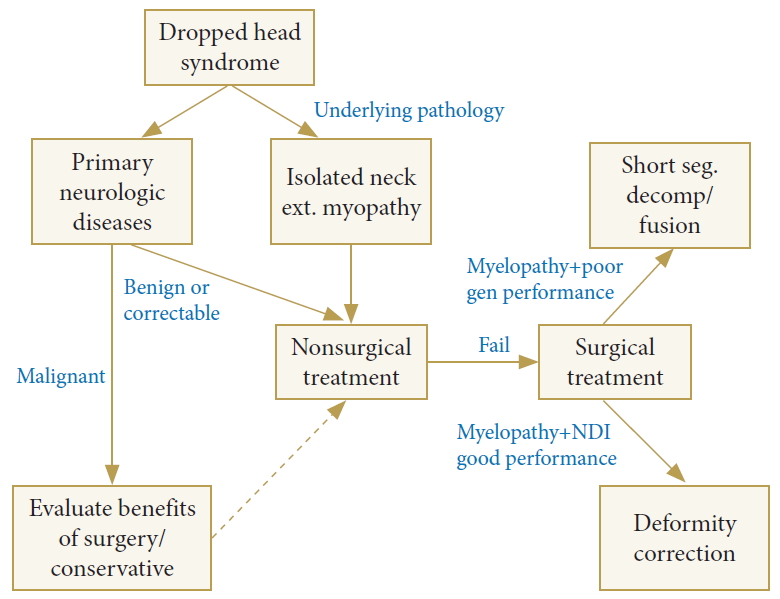
Fig. 6.
(A) Preoperative images in the hypokinetic hypotonic patient. (B) Postoperative x-rays in the hypokinetic hypotonic patient.

Table 1.
Scoliosis Research Society Classification of Neuromuscular Spinal Deformity [4]
Table 2.
Summary of conditions associated with dropped head deformity [53]
REFERENCES
1. McDonald CM. Clinical approach to the diagnostic evaluation of hereditary and acquired neuromuscular diseases. Phys Med Rehabil Clin N Am 2012 23:495-563.



2. Rose L, McKim D, Leasa D, et al. Trends in incidence, prevalence, and mortality of neuromuscular disease in Ontario, Canada: a population-based retrospective cohort study (2003-2014). PLoS One 2019 14:e0210574.



3. Mah JK, Korngut L, Fiest KM, et al. A systematic review and meta-analysis on the epidemiology of the muscular dystrophies. Can J Neurol Sci 2016 43:163-77.


4. Berven S, Bradford DS. Neuromuscular scoliosis: causes of deformity and principles for evaluation and management. Semin Neurol 2002 22:167-78.



5. Bar-On E, Floman Y, Sagiv S, et al. Orthopaedic manifestations of familial dysautonomia. A review of one hundred and thirty-six patients. J Bone Joint Surg Am 2000 82:1563-70.


6. Durr A, Cossee M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med 1996 335:1169-75.


7. Moon BJ, Smith JS, Ames CP, et al. Prevalence and type of cervical deformities among adults with Parkinson’s disease: a cross-sectional study. J Neurosurg Spine 2016 24:527-34.


8. Hyun SJ, Lee BH, Park JH, et al. Proximal junctional kyphosis and proximal junctional failure following adult spinal deformity surgery. Korean J Spine 2017 14:126-32.




9. Bae J, Lee SH. Minimally invasive spinal surgery for adult spinal deformity. Neurospine 2018 15:18-24.




10. Ohrt-Nissen S, Dahl B, Gehrchen M. Choice of rods in surgical treatment of adolescent idiopathic scoliosis: what are the clinical implications of biomechanical properties? – A review of the literature. Neurospine 2018 15:123-30.




11. Murphy RF, Mooney JF 3rd. Current concepts in neuromuscular scoliosis. Curr Rev Musculoskelet Med 2019 12:220-7.




12. Hensinger RN, MacEwen GD. Spinal deformity associated with heritable neurological conditions: spinal muscular atrophy, Friedreich's ataxia, familial dysautonomia, and Charcot-Marie-Tooth disease. J Bone Joint Surg Am 1976 58:13-24.


13. Gu Y, Shelton JE, Ketchum JM, et al. Natural history of scoliosis in nonambulatory spastic tetraplegic cerebral palsy. Pm R 2011 3:27-32.


14. Thometz JG, Simon SR. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg Am 1988 70:1290-6.


15. Shapiro F, Zurakowski D, Bui T, et al. Progression of spinal deformity in wheelchair-dependent patients with Duchenne muscular dystrophy who are not treated with steroids: coronal plane (scoliosis) and sagittal plane (kyphosis, lordosis) deformity. Bone Joint J 2014 96-B:100-5.


16. Roberto R, Fritz A, Hagar Y, et al. The natural history of cardiac and pulmonary function decline in patients with duchenne muscular dystrophy. Spine (Phila Pa 1976) 2011 36:E1009-17.



17. Galasko CS, Delaney C, Morris P. Spinal stabilisation in Duchenne muscular dystrophy. J Bone Joint Surg Br 1992 74:210-4.


18. Nishihara N, Tanabe G, Nakahara S, et al. Surgical treatment of cervical spondylotic myelopathy complicating athetoid cerebral palsy. J Bone Joint Surg Br 1984 66:504-8.


19. Wong AS, Massicotte EM, Fehlings MG. Surgical treatment of cervical myeloradiculopathy associated with movement disorders: indications, technique, and clinical outcome. J Spinal Disord Tech 2005 18 Suppl:S107-14.


20. White AA 3rd, Panjabi MM. The basic kinematics of the human spine. A review of past and current knowledge. Spine (Phila Pa 1976) 1978 3:12-20.


21. Obeso JA, Rodríguez-Oroz MC, Rodríguez M, et al. The basal ganglia and disorders of movement: pathophysiological mechanisms. News Physiol Sci 2002 17:51-5.


22. Kim H, Lee CK, Yeom JS, et al. Asymmetry of the cross-sectional area of paravertebral and psoas muscle in patients with degenerative scoliosis. Eur Spine J 2013 22:1332-8.




23. Shafaq N, Suzuki A, Matsumura A, et al. Asymmetric degeneration of paravertebral muscles in patients with degenerative lumbar scoliosis. Spine (Phila Pa 1976) 2012 37:1398-406.


24. Yagi M, Hosogane N, Watanabe K, et al. The paravertebral muscle and psoas for the maintenance of global spinal alignment in patient with degenerative lumbar scoliosis. Spine J 2016 16:451-8.


25. Saito N, Ebara S, Ohotsuka K, et al. Natural history of scoliosis in spastic cerebral palsy. Lancet 1998 351:1687-92.


26. Majd ME, Muldowny DS, Holt RT. Natural history of scoliosis in the institutionalized adult cerebral palsy population. Spine (Phila Pa 1976) 1997 22:1461-6.


27. Barbeau A, Duvoisin RC, Gerstenbrand F, et al. Classification of extrapyramidal disorders. Proposal for an international classification and glossary of terms. J Neurol Sci 1981 51:311-27.


29. Kang J, Hosogane N, Ames C, et al. Diversity in surgical decision strategies for adult spine deformity treatment: the effects of neurosurgery or orthopedic training background and surgical experience. Neurospine 2018 15:353-61.




30. Kyrölä KK, Salme J, Tuija J, et al. Intra- and interrater reliability of sagittal spinopelvic parameters on full-spine radiographs in adults with symptomatic spinal disorders. Neurospine 2018 15:175-81.




31. Wilson JRF, Badhiwala JH, Moghaddamjou A, et al. Degenerative cervical myelopathy; a review of the latest advances and future directions in management. Neurospine 2019 16:494-505.




32. Bajamal AH, Kim SH, Arifianto MR, et al. Posterior surgical techniques for cervical spondylotic myelopathy: WFNS Spine Committee Recommendations. Neurospine 2019 16:421-34.




33. Joaquim AF, Baum GR, Tan LA, et al. Dynamic cord compression causing cervical myelopathy. Neurospine 2019 16:448-53.




34. Parthiban J, Alves OL, Chandrachari KP, et al. Value of surgery and nonsurgical approaches for cervical spondylotic myelopathy: WFNS Spine Committee Recommendations. Neurospine 2019 16:403-7.




35. Contarino MF, Van Den Dool J, Balash Y, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol 2017 8:35.



37. Contarino MF, Smit M, van den Dool J, et al. Unmet needs in the management of cervical dystonia. Front Neurol 2016 7:165.



38. Castelão M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev 2017 12:Cd003633.


39. Kim KN, Ahn PG, Ryu MJ, et al. Long-term surgical outcomes of cervical myelopathy with athetoid cerebral palsy. Eur Spine J 2014 23:1464-71.



40. Vidailhet M. Treatment of movement disorders in dystoniachoreoathtosis cerebral palsy. Handb Clin Neurol 2013 111:197-202.


41. Kim HJ, Ahn HS, Yim SY. Effectiveness of surgical treatment for neglected congenital muscular torticollis: a systematic review and meta-analysis. Plast Reconstr Surg 2015 136:67e-77e.


42. Kim JH, Yum TH, Shim JS. Secondary cervicothoracic scoliosis in congenital muscular torticollis. Clin Orthop Surg 2019 11:344-51.



43. Lee GS, Lee MK, Kim WJ, et al. Adult patients with congenital muscular torticollis treated with bipolar release: report of 31 cases. J Korean Neurosurg Soc 2017 60:82-8.



44. Lake W, Shah H. Intrathecal baclofen infusion for the treatment of movement disorders. Neurosurg Clin N Am 2019 30:203-9.


45. Taira T, Ueta T, Katayama Y, et al. Rate of complications among the recipients of intrathecal baclofen pump in Japan: a multicenter study. Neuromodulation 2013 16:266. -72. discussion 72.


46. Motta F, Antonello CE. Analysis of complications in 430 consecutive pediatric patients treated with intrathecal baclofen therapy: 14-year experience. J Neurosurg Pediatr 2014 13:301-6.


47. Dru AB, Lockney DT, Vaziri S, et al. Cervical spine deformity correction techniques. Neurospine 2019 16:470-82.




48. Lee CK, Jeon HR, Yoon DH, et al. Clinical outcomes of correcting cervical deformity in cerebral palsy patients. World Neurosurg 2016 96:500-9.


49. Deora H, Kim SH, Behari S, et al. Anterior surgical techniques for cervical spondylotic myelopathy: WFNS Spine Committee Recommendations. Neurospine 2019 16:408-20.




50. Sharan AD, Kaye D, Charles Malveaux WM, et al. Dropped head syndrome: etiology and management. J Am Acad Orthop Surg 2012 20:766-74.


51. Endo K, Kudo Y, Suzuki H, et al. Overview of dropped head syndrome (Combined survey report of three facilities). J Orthop Sci 2019 24:1033-6.


52. Drain JP, Virk SS, Jain N, et al. Dropped head syndrome: a systematic review. Clin Spine Surg 2019 32:423-9.


53. Martin AR, Reddy R, Fehlings MG. Dropped head syndrome: diagnosis and management. Evid Based Spine Care J 2011 2:41-7.




54. Smith JS, Shaffrey CI, Kim HJ, et al. Prospective multicenter assessment of all-cause mortality following surgery for adult cervical deformity. Neurosurgery 2018 83:1277-85.



55. Etame AB, Wang AC, Than KD, et al. Outcomes after surgery for cervical spine deformity: review of the literature. Neurosurg Focus 2010 28:E14.

56. Toll BJ, Samdani AF, Janjua MB, et al. Perioperative complications and risk factors in neuromuscular scoliosis surgery. J Neurosurg Pediatr 2018 22:207-13.


57. Rumalla K, Yarbrough CK, Pugely AJ, et al. Spinal fusion for pediatric neuromuscular scoliosis: national trends, complications, and in-hospital outcomes. J Neurosurg Spine 2016 25:500-8.


58. Cognetti D, Keeny HM, Samdani AF, et al. Neuromuscular scoliosis complication rates from 2004 to 2015: a report from the Scoliosis Research Society Morbidity and Mortality database. Neurosurg Focus 2017 43:E10.

































