 |
 |
- Search
|
|
||
Abstract
Cervical spinal deformity (CSD) is a complex condition characterized by abnormal curvature and cervical spine alignment. It can lead to a multitude of symptoms, including chronic pain, neurological deficits, and functional impairments, severely impacting an individualŌĆÖs health-related quality of life (HRQoL). Surgical intervention is often necessary to address the deformity and alleviate symptoms, but optimal surgical strategies remain a topic of ongoing research and debate. This narrative review aims to provide an in-depth overview of the surgical management of CSD, focusing on optimizing patient outcomes and enhancing readersŌĆÖ understanding of the complexities involved. We begin by discussing the importance of preoperative assessment, including comprehensive radiographic evaluation and careful consideration of the global spinal alignment. The relationship between the cervical spine and the reciprocal changes that occur are explored to guide surgeons in their decision-making process. Furthermore, we delve into the selection of fusion levels, emphasizing the significance of identifying the primary driver of deformity. We review the current literature on optimal alignment targets and strategies to optimize surgical planning. By providing a comprehensive analysis of the surgical management of CSD, this review aims to enhance the readersŌĆÖ knowledge and assist surgeons in making informed decisions when planning and executing surgical interventions. Understanding the intricacies of CSD correction and the latest advancements in the field can ultimately improve patient outcomes and enhance HRQoL for individuals suffering from this challenging condition.
The primary role of the normal cervical spine is to carry the weight of the head and transmit it to the rest of the spine and body [1]. Additionally, it allows for the movement of the head and neck, enabling various motions while ensuring the protection of the spinal cord in the neck region. Furthermore, it aids in maintaining a horizontal gaze and provides structural support and stability [2]. However, when disrupted, these functions are compromised, leading to a profound negative impact on the individualŌĆÖs health-related quality of life (HRQoL) [1-3].
With causes ranging from congenital abnormalities to degenerative changes, traumatic injuries, and post-surgical complications, cervical spinal deformity (CSD) presents a complex and multifaceted problem [4]. CSD is a complex and challenging condition marked by aberrant curvature of the cervical spine, often leading to debilitating pain, neurological impairments, and physical deformities. It is commonly associated with neck pain, radiculopathy, myelopathy, changes in head position and horizontal gaze, dysphagia, and obstructive respiratory states [5,6]. Its profound impact on patientsŌĆÖ HRQoL underscores the urgent need for effective interventions.
Surgical correction of CSD is often necessary to alleviate symptoms and improve patient outcomes [4]. In recent years, advances in surgical techniques and instrumentation have made it possible to achieve successful correction of even the most severe CSDs. However, the correction of CSD is a complex and challenging surgical procedure that requires careful planning and execution. It is increasingly acknowledged that intricate and interconnected relationships exist between the sagittal curves of the entire spine and pelvis [7]. The presence of these compensatory mechanisms poses a significant challenge in achieving optimal correction of CSD.
This narrative review aims to provide a comprehensive overview of CSD surgical strategies with a specific focus on cervical kyphosis (CK). We will delve into the intricate surgical considerations, including radiographic parameters related to cervical deformity. Additionally, we will discuss the preoperative evaluation and fusion-level selection, highlighting their significance in ensuring adequate fixation and optimal surgical outcomes.
Traditionally, a balanced spine has been defined based on spinopelvic parameters, including the C7 plumb line (C7PL). The C7PL is commonly used as a surrogate for assessing sagittal trunk balance, as it is typically consistent with the global alignment of the spine in the general population and serves as a practical tool for estimating sagittal trunk balance [8,9]. However, it should be noted that a spine can appear balanced (compensated), yet still have inadequate spinopelvic parameters [10]. Even though C7PL is a convenient method for estimating sagittal balance, its discrepancy with the global alignment has been widely recognized [8,11].
When the distance between the gravity line, defined as the vertical line from the ear canal, and the C7PL exceeds 30 mm, it is referred to as occiput-trunk discordance [8]. This discordance hinders the accurate assessment of true global spinal alignment using the C7PL alone [12]. In patients with CSD, achieving concordance between the occiput and trunk is challenging due to the limited ability to extend the cervical spine [13]. Therefore, a posterior shifting of the C7PL is necessary to optimize the positioning of the head [13,14]. Subsequently, compensation in thoracolumbar alignment occurs as a result of the posterior shifting of the C7PL [13] (Fig. 1). Those with a posterior shifting of C7PL from the pelvis are grouped as head-balanced (i.e., compensated CK), and those with C7PL on the pelvis as trunk-balanced (i.e., decompensated CK) [13].
Ha et al. [7] described the reciprocal changes in cervical spine following thoracolumbar deformity surgery. Reciprocal changes in cervical lordosis (CL), C2ŌĆō7 sagittal vertical axis (cSVA), and T1 slope (T1S) have been observed. (Figs. 2, 3) In a similar manner, studies have reported that correcting severe cervical kyphotic deformity, defined as a cSVA greater than 40 mm, has an impact on thoracolumbar decompensation down to the lumbar spine [13-15]. However, in cases of mid-CK correction, postoperative decompensation is primarily observed in thoracic segment where as in severe deformities, lumbar decompensation is evidenced by a reduction in lumbar lordosis (LL) and an associated increase in the mismatch between pelvic incidence (PI) and LL [14]. Following cervical reconstruction, compensatory changes in the upper cervical and thoracolumbar spine resolve reciprocally, albeit in different patterns [14-16] (Fig. 4).
The clinical significance of these reciprocal changes has been highlighted by Hyun et al. [15], who reported notable improvements in HRQoL scores following cervical reconstruction, particularly in cases where global sagittal alignment was restored, and there was concordance between the occiput and trunk (head-balanced). In the head-balanced group, cervical realignment surgery led to significant enhancements not only in reductions in visual analogue scale scores for back pain and improvements in Oswestry Disability Index scores, but also in thoracolumbar spine radiological indices, marked by a decrease in the PIŌĆōLL mismatch [15].
Considering these factors, the concept of global balance utilizing global alignment has been introduced to assess patients undergoing cervical realignment surgery. By incorporating the global alignment, which takes into account the entire spinal column, a more comprehensive evaluation of the patientŌĆÖs spinal alignment can be achieved, leading to improved surgical planning and outcomes [17]. Surgeons should be aware of these reciprocal changes and may consider longer fusion into the lumbar spine, especially in patients with severe cervical and preexisting thoracolumbar deformities [13,14]. This knowledge can guide surgical decision-making and facilitate comprehensive correction of the complex interactions between the cervical, thoracic, and lumbar regions, ultimately improving patient outcomes.
Due to the considerations mentioned above, conducting a comprehensive evaluation of patients with CSD necessitates the utilization of full-length standing radiographs. This approach is of paramount importance in order to identify potential concurrent thoracolumbar and cervical pathologies [18]. It has been reported that up to 53% of patients presenting with thoracolumbar deformity exhibit accompanying conditions such as CK or an increased cSVA [19]. Thus, the implementation of full-length standing radiographs facilitates a more comprehensive assessment of the spinal alignment and aids in identifying coexisting deformities, ensuring a comprehensive understanding of the complex nature of CSD [18,20]. Understanding the compensatory mechanisms is paramount for successful cervical realignment surgery [21].
Identifying the reciprocal changes occurring in the thoracic and lumbar spine as a result of primary cervical pathology is crucial, as identifying and addressing the primary driver of CSD during fusion procedures is vital in achieving optimal radiological and clinical outcomes [18,20]. It has been reported that patients with a primary cervical apex sagittal deformity had the best radiological outcomes when the cervical driver of deformity was specifically addressed [20]. Conversely, patient improvement was limited if the cervical deformity was driven by a thoracic apex sagittal deformity that was not treated [18,20].
Patients with distal junctional kyphosis (DJK) exhibited higher T1SŌĆōCL, CL, cSVA, C2ŌĆōT3 angle, and C2 slope at baseline and at a 1-year follow-up, indicative of either a thoracic driver of deformity or a more severe cervical deformity [20,22]. DJK is defined by a postoperative change in kyphosis greater than 10┬░ between the lowest instrumented vertebra (LIV) and the vertebra 2 levels below the LIV [23,24]. However, unlike proximal junctional kyphosis angle in thoracolumbar deformity, there is currently a lack of studies validating the significance of the DJK angle below the LIV in adjacent cervical deformity.
Fusion-level selection in CSD remains an area of ongoing debate and lacks a strong consensus among surgeons. The selection of surgical approach, osteotomies, number of fusion levels, and the determination of upper instrumented vertebra (UIV) and LIV levels exhibit remarkable variability, as highlighted by Smith et al. [25] Despite the growing understanding of the complexity and diverse manifestations of CSD, the development of DJK following cervical realignment surgery remains a significant postoperative concern. LIV selection is considered a key component in preventing DJK, aiming to avoid the curveŌĆÖs apex [26]. However, the relationship between DJK and the LIV after cervical realignment surgery has received limited attention in the literature [22,23,27].
When performing multilevel cervical arthrodesis, spine surgeons often face the dilemma of whether to extend the fusion beyond the cervicothoracic junction (CTJ) [28,29]. The advantage of not crossing the CTJ is preserving the physiological range of motion at the C7ŌĆōT1 segment [30]. However, terminating the fusion at C7 is associated with several risks. Studies have shown that ending a multilevel cervical construct at C7 increases the likelihood of revision surgery or distal junctional problems compared to extending the construct to T1 [31,32]. Furthermore, stopping the fusion at C7 is associated with suboptimal postoperative sagittal alignment, characterized by increased cSVA, greater T1S, and larger T1SŌĆōCL [33].
On the other hand, crossing the CTJ has its potential benefits. By anchoring the arthrodesis to the rigid structure of the thoracic spine, sagittal stability of the construct can be achieved, enabling more significant correction or prevention of sagittal deformities [34]. However, this approach is not without drawbacks. A multicenter retrospective study indicated that extending the fusion to T1 was associated with higher intraoperative blood loss, longer surgery duration, and prolonged hospital stay, although it demonstrated a lower rate of pseudarthrosis [35]. Despite these challenges, the advantages of crossing the CTJ, such as preventing distal junctional problems and improving deformity correction, appear to outweigh the slightly increased invasiveness of the surgical procedure, particularly in patients with mid-CK. These results favor selecting the LIV extending into the thoracic spine. These findings were consistent with a study by Ye et al. [24], which also suggested that the risk of DJK is lower when the LIV is at the upper thoracic segment compared to the lower cervical segment. Additionally, DJK incidence was found to be highest when the LIV level was at the lower thoracic or thoracolumbar junction.
A study by Kim et al. [27] compared CSD patients with LIV at the CTJ versus the proximal thoracic (PT) spine. The results revealed that patients with fusion to the PT spine demonstrated larger cSVA malalignment, greater T1SŌĆōCL mismatch, and a higher likelihood of requiring a pedicle subtraction osteotomy for correction. Moreover, the PT group experienced a greater correction of cSVA, more reciprocal change of thoracic kyphosis (TK), higher final TK and DJK angles, and a higher incidence of DJK and reoperation, although statistical significance was not reached.
A novel CSD classification was devised by Koller et al. [36,37] and the European Cervical Spine Research Society (ECSRS). The Koller-ECSRS classification system establishes a treatment-guiding algorithm specifically designed for patients with rigid CK. Through a retrospective, multicenter review of patients with this condition, Koller-ECSRS categorized the deformity into 4 types based on the regional and global state of balance, designated as types A, B, C, and D. Type A represented patients with cervical/cervicothoracic kyphosis and maintained global balance. Type B encompassed those with cervical/cervicothoracic kyphotic deformities and concomitant global imbalance. Type C corresponded to cervicothoracic kyphotic deformities with inadequate compensatory CL and persistent global imbalance. Type D included patients with appropriate CL and maintained global balance. The Koller-ECSRS classification demonstrated the capacity to categorize cervical balance and alignment while concurrently reflecting the global balance of patients with rigid CK. Despite the utility of the Koller-ECSRS classification in providing a comprehensive assessment of CSD, it is important to note certain limitations. The classification offers the most common surgical techniques utilized by the cohort of surgeons involved in the study, and there was limited discussion on further guidelines for surgical planning based on the specific classification types [38]. As such, while the classification offers valuable insights into regional and global balance in CSD, additional research was necessary to develop comprehensive surgical strategies based on the different classification types including fusion-level selection.
Recently, a more refined and individualized surgical strategy based on the Kim-ISSG (International Spine Study Group) classification has been proposed [38-40]. The Kim-ISSG classification is the first classification schema to adapt dynamic cervical radiographs in defining CSD [38]. The Kim-ISSG classification identifies 4 distinct types of cervical spinal deformities: (1) ŌĆśflat neckŌĆÖ deformities, (2) focal kyphotic deformities, (3) cervicothoracic deformities, and (4) coronal deformities. Each type of deformity presents unique challenges and considerations in the context of preoperative planning and surgical intervention. A recent study by Kim et al. [39] described the potential surgical strategy for each subtype of CSD. The UIV selected did not significantly vary across cervical subtypes. There was, however, a significant difference in the LIV selected across subtypes. Type 3 cervicothoracic deformity patients had less upper thoracic LIV and more thoracolumbar LIV. For patients with type 2 focal deformity, there were significantly more patients with a LIV in the cervical spine. This approach may provide valuable insight on a selection of approaches for patients with CSD. Ultimately, however, the fusion length will also depend on the magnitude of the deformity, the location of the deformity, and the presence/absence of concurrent degeneration at the adjacent segments in the planned end vertebrae.
ItŌĆÖs worth mentioning that several classification systems exist, each with its distinct advantages and applicability. The field of CSD is dynamic, and a holistic approach often requires the amalgamation of insights from multiple classification frameworks. The inclusion of Kim-ISSG in our manuscript serves as an illustrative example and shouldnŌĆÖt be construed as an endorsement of its superiority.
While CK was traditionally deemed abnormal, recent studies have revealed that CK may not always represent an abnormal alignment, particularly in patients with low TK [2,41,42]. However, it is important to consider the curvature of the C2ŌĆō7 segment in patients with functional neck disability.
A significant predictor of disability in cervical malalignment is the cSVA. Pioneering work by Tang et al. [3] highlighted the relationship between various radiographic parameters and HRQoL in postoperative patients who underwent a long-segment posterior cervical fusion. Increasing cSVA was significantly correlated to increasing disability on the Neck Disability Index (NDI) and the 36-item Short Form health survey physical composite score [3]. Several studies have since corroborated this finding [43-45]. Subsequent studies have consistently supported this correlation, demonstrating that increasing cSVA is associated with greater disability scores. In particular, Hyun et al. [44] proposed that a cSVA greater than 40- and 70-mm corresponds to moderate- (NDI> 25) and severe disability (NDI> 35), respectively.
In addition to cSVA, the T1 slope minus CL (T1SŌĆōCL) has emerged as another important parameter related to cervical disability [46]. Similar to the PIŌĆōLL mismatch observed in thoracolumbar deformity, the T1SŌĆōCL mismatch is gaining recognition as a local regulator of cervical alignment. T1S strongly correlates with TK, CL, and the C0ŌĆō2 angle [47,48]. Functionally, a substantial T1S necessitates a corresponding CL to achieve harmonious alignment and facilitate horizontal gaze. Insufficient CL in this context results in a high T1SŌĆōCL, adversely impacting disability scores [49]. Oe et al. [50] conducted a study on 656 volunteers aged 50 to 89 years and found that cSVA> 40 mm or more, T1S> 40┬░, and T1SŌĆōCL> 20┬░ had worse EuroQoL-5 dimension health status scores. Iyer et al. [45] were among the first to show the impact that T1SŌĆōCL can have on disability scores. In their analysis of preoperative patients, higher T1SŌĆōCL, along with cSVA, was found to be an independent predictor of increased disability [45]. Hyun et al. [44] found a similar relationship in postoperative patients undergoing multilevel posterior surgery that a T1SŌĆōCL exceeding approximately 20┬░ and 25┬░ was associated with moderate- and severe disability, respectively.
Recent research has also focused on C2 slope (C2S), defined as the angle between the C2 endplate and a horizontal line, as an approximation of T1SŌĆōCL (Fig. 2). C2S has shown strong correlations with various measures of CSD, including cSVA and T1SŌĆōCL, and moderate correlations with HRQoL measures [17,51-53]. Notably, Protopsaltis et al. [51] proposed that a C2S of 20┬░ is indicative of moderate disability. Another study identified optimal thresholds for T1S and C2S [52]. Reducing T1S below the severe threshold of 45.5┬░ and further below the optimal threshold of 26┬░ substantially reduces the occurrence of distal junctional failure. Patients with C2S exceeding the severe threshold of 38┬░ failed to achieve optimal clinical outcomes. Overall 60% of the patients developed DJK, and 85.7% underwent reoperation [52]. Notably, patients above the severe threshold for C2S had poor outcomes.
Based on these significant findings, an individualized cervical realignment strategy has been recently proposed, with a primary focus on correcting C2S and LIV selection [54]. A hierarchical approach recommends first correcting C2S to below 10┬░, followed by selecting a stable LIV, defined as the LIV with an inclination angle (Fig. 5) ranging from 0┬░ to 40┬░, and finally achieving correction of cSVA below 35 mm (Fig. 6). The LIV inclination is defined as the angle between the vertical plumb line and the best-fit line crossing the center of the LIV, LIV-1, and LIV-2. Williamson et al. [54] sought to devise a stepwise approach by prioritizing cervical parameters in order to address cervical realignment surgery to improve clinical outcomes and reduce DJK and reoperation. The study revealed through adjusted analysis using the analysis of covariance and multivariable regression that this hierarchical approach led to a significantly lower rate of poor clinical outcomes. Implementation of this approach has demonstrated superior 2-year HRQoL outcomes, reduced rates of DJK and reoperation, and higher rates of attaining optimal outcomes [54]. There are limitations that this hierarchical approach has not been confirmed in other studies. Despite the limitations, this is the first attempt to provide stepwise approach to cervical realignment surgery by prioritizing cervical parameters. These findings underscore the possible importance of addressing C2S during cervical realignment surgery, selecting an appropriate LIV, and attaining the ideal cSVA threshold.
In our current practice, we adopt a systematic approach aligned with the structure of this article. Our assessment commences with a thorough global evaluation of compensatory mechanisms and the identification of primary deformity drivers. The current assessment of identifying the primary driver of deformity lacks a straightforward algorithm and often relies on expert consensus only [20,22]. We endeavor to infuse this process with as much objectivity as possible.
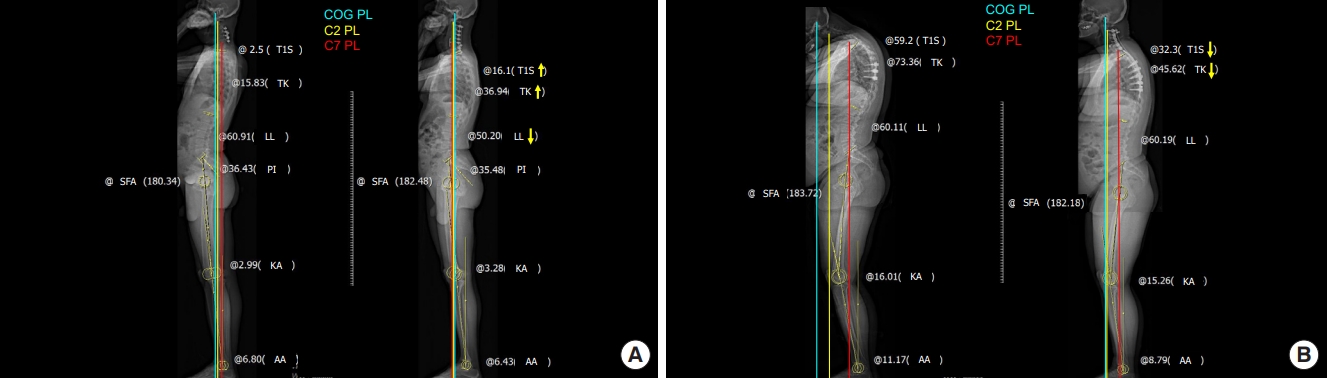
To achieve this, we have divided our patient cohort into 2 distinct subgroups: compensated and decompensated cases (Fig. 1) [15]. This categorization assists in delineating patients who retain a balanced state (akin to Koller-ECSRS type A or Kim-ISSG classification focal kyphosis) from those who manifest more pronounced cervical malalignment (similar to Koller-ECSRS type B or C or Kim-ISSG classification flat neck or cervicothoracic). By anchoring our LIV selection to this classification, we bring into focus the nuanced demands of each case.
In the compensated CK scenario, where global balance is retained, we generally select the LIV at the C7 or PT level, depending on the magnitude of correction (Fig. 4A). On the other hand, the decompensated patients, while similar in some aspects, often resemble Koller-ECSRS type B or C or Kim-ISSG classification flat neck or cervicothoracic (Fig. 4B). In these instances, we adopt an approach that extends the LIV beyond the PT spine while avoiding the apex, which is in line with the recommendations set forth by Williamson et al. [54]. This stratagem harmonizes with our objective of integrating the global compensatory status, thereby mitigating potential complications or malalignment issues stemming from cervical realignment surgery.
Subsequently, we proceed with preoperative planning, encompassing the determination of adjustment targets. Our focus lies on individualized optimization of T1S, C2S, and cSVA, while considering potential reciprocal changes that might compromise the patientŌĆÖs disability, including horizontal gaze. We then design the overall CTJ angle. Intraoperatively, our emphasis remains on optimal positioning. We maximize head elevation to minimize cSVA (< 4 cm) and reduce excessive T1S (T1SŌĆōCL< 20┬░), thereby enhancing global and regional balance. Intraoperative adjustments and angular corrections are tailored to each patientŌĆÖs unique requirements.
We recognize that the process of selecting an appropriate LIV for CSD surgery is multifaceted, incorporating variables such as the extent of deformity and existing malalignment. While the optimal fusion levels for treating CSD remain enigmatic, the cornerstone of our recommendations lies in the significance of meticulous preoperative planning. Identifying primary drivers of deformity bears paramount importance in guiding the decision to extend fusion levels into the mid to lower thoracic spine. While we provide these recommendations based on the culmination of our experiences and the insights gathered from this narrative review, we acknowledge the ongoing need for prospective studies to establish an evidence-based approach for fusion-level selection in CSD.
This integrated approach aims to serve as a practical guide for clinicians navigating the intricate landscape of CSD assessment and surgical planning. By implementing these recommendations, we aspire to enhance patient outcomes and contribute to the ongoing advancements in this field.
The surgical correction of CSD is a complex and challenging procedure that requires careful consideration of various radiographic parameters and individualized treatment strategies. By incorporating global balance, addressing reciprocal changes, and selecting fusion levels based on the primary driver of deformity, surgeons can optimize surgical outcomes and improve the HRQoL of patients with CSD. Continued research and advancements in surgical techniques will further enhance our understanding and management of this intricate condition.
NOTES
Fig.┬Ā1.
Classification of cervical kyphosis based on compensatory mechanism and occiput-trunk concordance. PL, plumb line; CK, cervical kyphosis; PI, pelvic incidence; LL, lumbar lordosis; TK, thoracic kyphosis.
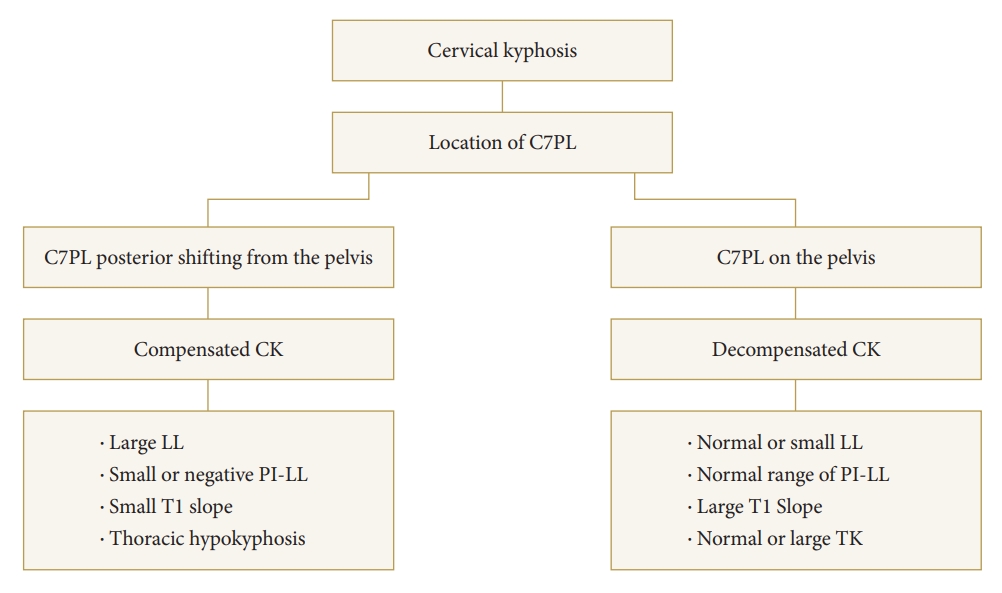
Fig.┬Ā2.
Schematic illustration of cervical parameters. C2ŌĆō7 lordosis (CL) was measured as the angle of intersection between lines parallel to the inferior endplates of C2 and C7. C2ŌĆō7 sagittal vertical axis (cSVA) was defined as the deviation of the C2 plumb line (extending from the centroid of the C2 vertebra) from the posterior superior end plate of C7. T1 slope (T1S) was defined as an angle formed between the T1 upper endplate and the horizontal line. C2 slope (C2S) was defined as the angle between the C2 endplate and a horizontal line. C2S is a mathematical approximation of T1SŌĆōCL when T1S and C7 slope are approximately equivalent.
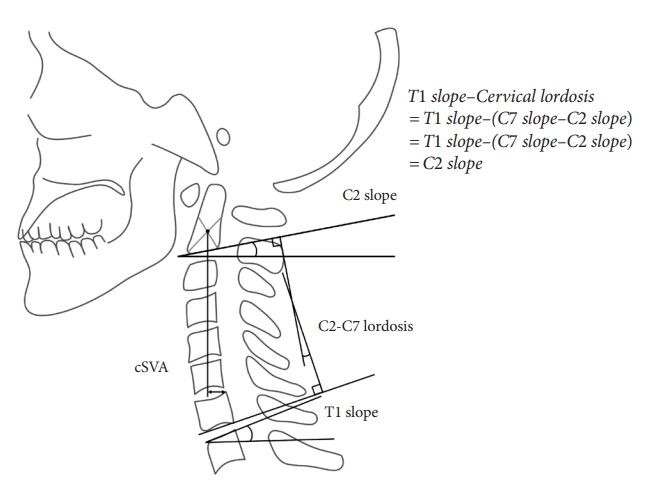
Fig.┬Ā3.
Reciprocal changes following thoracolumbar deformity surgery. Note the decrease in cervical hyperlordosis, decreased T1 slope, and C2ŌĆō7 sagittal vertical axis.
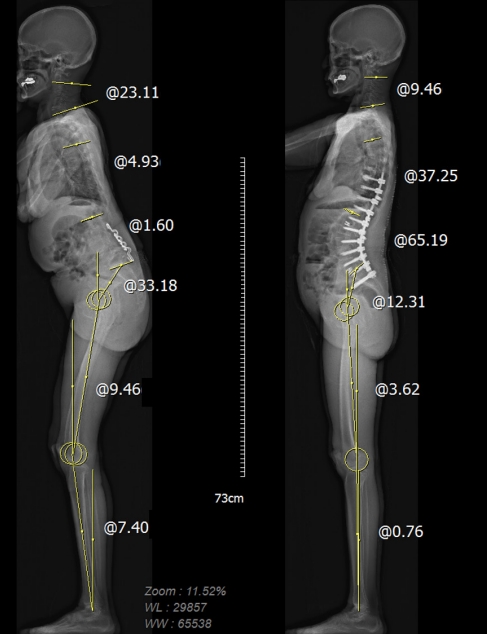
Fig.┬Ā4.
(A) Radiograph of a head-balanced (compensated) cervical kyphosis patient. The COG PL is located on the femoral head, indicating overall balance, but the C7PL is located markedly posteriorly, indicating cervical malalignment. The patientŌĆÖs cervical deformity was corrected to achieve global sagittal balance and concordance between the OT. However, there were no significant changes in lumbopelvic alignment or lower-extremity alignment parameters. (B) Radiograph of a trunk-balanced (decompensated) cervical kyphosis patient. The COG PL is located markedly anteriorly, indicating imbalance, but the C7PL is located on the femoral head. Before surgery, the sagittal plumb lines indicate cervical sagittal imbalance and discordance between the occiput and trunk. No significant changes were found in lumbopelvic alignment or the lower-extremity alignment parameters. COG, center of gravity; PL, plumb line; OT, occiput-trunk; T1S, T1 slope; TK, thoracic kyphosis; LL, lumbar lordosis; PI, pelvic incidence; KA, knee angle; AA, ankle angle.
REFERENCES
1. Dru AB, Lockney DT, Vaziri S, et al. Cervical spine deformity correction techniques. Neurospine 2019;16:470-82.




2. Diebo BG, Challier V, Henry JK, et al. Predicting cervical alignment required to maintain horizontal gaze based on global spinal alignment. Spine (Phila Pa 1976) 2016;41:1795-800.



3. Tang JA, Scheer JK, Smith JS, et al. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery 2012;71:662-9. discussion 669.



4. Smith JS, Line B, Bess S, et al. The health impact of adult cervical deformity in patients presenting for surgical treatment: comparison to united states population norms and chronic disease states based on the EuroQuol-5 dimensions questionnaire. Neurosurgery 2017;80:716-25.


5. Lamartina C, Berjano P. Classification of sagittal imbalance based on spinal alignment and compensatory mechanisms. Eur Spine J 2014;23:1177-89.



6. Gusi N, Olivares P, Rajendram R. The EQ-5D health-related quality of life questionnaire. Handbook of disease burdens and quality of life measures. In: Preedy VR, Watson RRet al., editors. Handbook of disease burdens and quality of life measures. New York: Springer; 2010. p. 87-99.
7. Ha Y, Schwab F, Lafage V, et al. Reciprocal changes in cervical spine alignment after corrective thoracolumbar deformity surgery. Eur Spine J 2014;23:552-9.




8. Yagi M, Takeda K, Machida M, et al. Discordance of gravity line and C7PL in patient with adult spinal deformity--factors affecting the occiput-trunk sagittal discordance. Spine J 2015;15:213-21.


9. Lafage V, Schwab F, Patel A, et al. Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine (Phila Pa 1976) 2009;34:E599-606.

10. Le Huec JC, Thompson W, Mohsinaly Y, et al. Sagittal balance of the spine. Eur Spine J 2019;28:1889-905.



11. Schwab F, Lafage V, Boyce R, et al. Gravity line analysis in adult volunteers: age-related correlation with spinal parameters, pelvic parameters, and foot position. Spine (Phila Pa 1976) 2006;31:E959-67.

12. Sugrue PA, McClendon J Jr, Smith TR, et al. Redefining global spinal balance: normative values of cranial center of mass from a prospective cohort of asymptomatic individuals. Spine (Phila Pa 1976) 2013;38:484-9.

13. Mizutani J, Verma K, Endo K, et al. Global spinal alignment in cervical kyphotic deformity: the importance of head position and thoracolumbar alignment in the compensatory mechanism. Neurosurgery 2018;82:686-94.



14. Mizutani J, Strom R, Abumi K, et al. how cervical reconstruction surgery affects global spinal alignment. Neurosurgery 2019;84:898-907.



15. Hyun SJ, Kim KJ, Jahng TA. The differential effect of cervical kyphosis correction surgery on global sagittal alignment and health-related quality of life according to head- and trunk-balanced subtype. J Neurosurg Spine 2021;34:839-48.


16. Lee JK, Hyun SJ, Yang SH, et al. Reciprocal changes following cervical realignment surgery. Neurospine 2022;19:853-61.




17. Kim CW, Hyun SJ, Kim KJ. Surgical impact on global sagittal alignment and health-related quality of life following cervical kyphosis correction surgery: systematic review. Neurospine 2020;17:497-504.




18. Passias PG, Jalai CM, Lafage V, et al. Primary drivers of adult cervical deformity: prevalence, variations in presentation, and effect of surgical treatment strategies on early postoperative alignment. Neurosurgery 2018;83:651-9.



19. Smith JS, Lafage V, Schwab FJ, et al. Prevalence and type of cervical deformity among 470 adults with thoracolumbar deformity. Spine (Phila Pa 1976) 2014;39:E1001-9.


20. Passias PG, Bortz C, Horn S, et al. Drivers of cervical deformity have a strong influence on achieving optimal radiographic and clinical outcomes at 1 year after cervical deformity surgery. World Neurosurg 2018;112:e61-8.


21. Nunez-Pereira S, Hitzl W, Bullmann V, et al. Sagittal balance of the cervical spine: an analysis of occipitocervical and spinopelvic interdependence, with C-7 slope as a marker of cervical and spinopelvic alignment. J Neurosurg Spine 2015;23:16-23.


22. Passias PG, Horn SR, Oh C, et al. Predicting the occurrence of postoperative distal junctional kyphosis in cervical deformity patients. Neurosurgery 2020;86:E38-46.



23. Passias PG, Vasquez-Montes D, Poorman GW, et al. Predictive model for distal junctional kyphosis after cervical deformity surgery. Spine J 2018;18:2187-94.


24. Ye J, Rider SM, Lafage R, et al. Distal junctional kyphosis in adult cervical deformity patients: where does it occur? Eur Spine J 2023;32:1598-606.



25. Smith JS, Klineberg E, Shaffrey CI, et al. Assessment of surgical treatment strategies for moderate to severe cervical spinal deformity reveals marked variation in approaches, osteotomies, and fusion levels. World Neurosurg 2016;91:228-37.


26. Lau D, Joshi RS, Haddad AF, et al. Incidence and risk factors of mechanical complications after posterior-based osteotomies for correction of moderate to severe adult cervical deformity: 1-year and 2-year follow-up. Neurosurgery 2022;90:207-14.


27. Kim HJ, Yao YC, Bannwarth M, et al. Cervicothoracic versus proximal thoracic lower instrumented vertebra have comparable radiographic and clinical outcomes in adult cervical deformity. Global Spine J 2023;13:1056-63.




28. Fayed I, Toscano DT, Triano MJ, et al. Crossing the cervicothoracic junction during posterior cervical decompression and fusion: is it necessary? Neurosurgery 2020;86:E544-50.



29. Okamoto N, Kato S, Doi T, et al. Relative risks and benefits of crossing the cervicothoracic junction during multilevel posterior cervical fusion: a multicenter cohort. World Neurosurg 2021;153:e265-74.


30. Bechara BP, Bell KM, Hartman RA, et al. In vivo analysis of cervical range of motion after 4- and 5-level subaxial cervical spine fusion. Spine (Phila Pa 1976) 2012;37:E23-9.


31. Osterhoff G, Ryang YM, von Oelhafen J, et al. Posterior multilevel instrumentation of the lower cervical spine: is bridging the cervicothoracic junction necessary? World Neurosurg 2017;103:419-23.


32. Schroeder GD, Kepler CK, Kurd MF, et al. Is it necessary to extend a multilevel posterior cervical decompression and fusion to the upper thoracic spine? Spine (Phila Pa 1976) 2016;41:1845-9.


33. Choi SJ, Suk KS, Yang JH, et al. What is a right distal fusion level for prevention of sagittal imbalance in multilevel posterior cervical spine surgery: C7 or T1? Clin Spine Surg 2018;31:441-5.


34. Rajjoub R, Michalopoulos GD, El Sammak S, et al. Crossing the cervicothoracic junction in multilevel cervical arthrodesis: a systematic review and meta-analysis. World Neurosurg 2022;162:e336-46.


35. Truumees E, Singh D, Geck MJ, et al. Should long-segment cervical fusions be routinely carried into the thoracic spine? A multicenter analysis. Spine J 2018;18:782-7.


36. Koller H, Ames C, Mehdian H, et al. Characteristics of deformity surgery in patients with severe and rigid cervical kyphosis (CK): results of the CSRS-Europe multi-centre study project. Eur Spine J 2019;28:324-44.



37. Koller H, Koller J, Mayer M, et al. Osteotomies in ankylosing spondylitis: where, how many, and how much? Eur Spine J 2018;27:70-100.



39. Kim HJ, Virk S, Elysee J, et al. Surgical strategy for the management of cervical deformity is based on type of cervical deformity. J Clin Med 2021;10:4826.



40. Kim HJ, Virk S, Elysee J, et al. The morphology of cervical deformities: a two-step cluster analysis to identify cervical deformity patterns. J Neurosurg Spine 2019 Nov 15:1-7. doi: 10.3171/2019.9.SPINE19730. [Epub].

41. Lee HJ, Kim IS, Hong JT. Physiologic cervical alignment change between cervical spine x-ray and computed tomography. J Korean Neurosurg Soc 2021;64:784-90.




42. Lee SH, Hyun SJ, Jain A. Cervical sagittal alignment: literature review and future directions. Neurospine 2020;17:478-96.




43. Bao H, Varghese J, Lafage R, et al. Principal radiographic characteristics for cervical spinal deformity: a health-related quality-of-life analysis. Spine (Phila Pa 1976) 2017;42:1375-82.


44. Hyun SJ, Kim KJ, Jahng TA, et al. Clinical impact of T1 slope minus cervical lordosis after multilevel posterior cervical fusion surgery: a minimum 2-year follow up data. Spine (Phila Pa 1976) 2017;42:1859-64.

45. Iyer S, Nemani VM, Nguyen J, et al. Impact of cervical sagittal alignment parameters on neck disability. Spine (Phila Pa 1976) 2016;41:371-7.


46. Jeon SI, Hyun SJ, Han S, et al. Relationship between cervical sagittal alignment and patient outcomes after anterior cervical fusion surgery involving 3 or more levels. World Neurosurg 2018;113:e548-54.


47. Lee SH, Kim KT, Seo EM, et al. The influence of thoracic inlet alignment on the craniocervical sagittal balance in asymptomatic adults. J Spinal Disord Tech 2012;25:E41-7.


48. Lee SH, Son ES, Seo EM, et al. Factors determining cervical spine sagittal balance in asymptomatic adults: correlation with spinopelvic balance and thoracic inlet alignment. Spine J 2015;15:705-12.


49. Lee JK, Hyun SJ, Yang SH, et al. Clinical impact and correlations of odontoid parameters following multilevel posterior cervical fusion surgery. Neurospine 2022;19:912-20.




50. Oe S, Togawa D, Nakai K, et al. the influence of age and sex on cervical spinal alignment among volunteers aged over 50. Spine (Phila Pa 1976) 2015;40:1487-94.


51. Protopsaltis TS, Ramchandran S, Tishelman JC, et al. The importance of C2 slope, a singular marker of cervical deformity, correlates with patient-reported outcomes. Spine (Phila Pa 1976) 2020;45:184-92.


52. Passfall L, Williamson TK, Krol O, et al. Do the newly proposed realignment targets for C2 and T1 slope bridge the gap between radiographic and clinical success in corrective surgery for adult cervical deformity? J Neurosurg Spine 2022 Apr 15:1-8. doi: 10.3171/2022.2.SPINE211576. [Epub].


- TOOLS





























