 |
 |
- Search
|
|
||
Abstract
Objective
Studies discussed few risk factors for specific patients, such as duration of disease; or surgical factors, such as duration and time of surgery; or C3 or C7 involvement, which could have led to the formation of hematomas (HTs). To investigate the incidence, risk factors especially the factors mentioned above, and management of postoperative HTs following anterior cervical decompression and fusion (ACF) for degenerative cervical diseases.
Methods
Medical records of 1,150 patients who underwent ACF for degenerative cervical diseases at our hospital between 2013 and 2019 were identified and reviewed. Patients were categorized into the HT group (HT group) or normal group (no-HT group). Demographic, surgical and radiographic data were recorded prospectively to identify risk factors for HT.
Results
Postoperative HT was identified in 11 patients, with an incidence rate of 1.0% (11 of 1,150). HT occurred within 24 hours postoperatively in 5 patients (45.5%), while it occurred at an average of 4 days postoperatively in 6 patients (54.5%). Eight patients (72.7%) underwent HT evacuation; all patients were successfully treated and discharged. Smoking history (odds ratio [OR], 5.193; 95% confidence interval [CI], 1.058–25.493; p = 0.042), preoperative thrombin time (TT) value (OR, 1.643; 95% CI, 1.104–2.446; p = 0.014) and antiplatelet therapy (OR, 15.070; 95% CI, 2.663–85.274; p = 0.002) were independent risk factors for HT. Patients with postoperative HT had longer days of first-degree/intensive nursing (p < 0.001) and greater hospitalization costs (p = 0.038).
Conclusion
Smoking history, preoperative TT value and antiplatelet therapy were independent risk factors for postoperative HT following ACF. High-risk patients should be closely monitored through the perioperative period. Postoperative HT in ACF was associated with longer days of first-degree/intensive nursing and more hospitalization costs.
Anterior cervical decompression and fusion (ACF) has been widely adopted by orthopedic surgeons and regarded as the classical gold standard for anterior cervical surgery [1-6]. These procedures often achieve very good surgical results with relatively low rates of associated complications [7,8]. Postoperative hematoma (HT) is a rare complication after ACF that can lead to acute airway obstruction (AAO), paralysis and even life-threatening conditions. Therefore, postoperative HTs must be quickly identified, and effective action should be undertaken to prevent catastrophic consequences. Some large studies have shown that the incidence of postoperative HT ranges from 0.4% to 5.6% [9-18]. The reported incidence of AAO caused by HT ranges from 0.2 to 1.9% [18].
Because the potential consequences of this complication are so severe, early detection and systematic evaluation of risk factors for HT are essential. If independent risk factors for HT can be identified, surgeons can explain the harm of postoperative HT and the necessity of intratracheal intubation to specific patients before surgery, undertake preventative measures during surgery to prevent the occurrence of postoperative HT, and then particular patients who might undergo close monitoring and intensive care could be identified after surgery. Therefore, identifying risk factors for HT is critical for both patients and clinicians.
Although there is considerable experience with ACF in the literature, these studies discussed few risk factors for specific patients, such as duration of disease; or surgical factors, such as duration and time of surgery; or C3 or C7 involvement, which could have led to the formation of HT. A total of 1,150 patients who underwent ACF for degenerative cervical diseases at our institution were identified and reviewed to investigate the incidence, risk factors, and management of postoperative HT following ACF.
This study was performed in the General Hospital of Northern Theater Command, a tertiary hospital in northeastern China. After obtaining approval from the Ethics Board of General Hospital of Northern Theater Command, we retrospectively reviewed the medical records of patients who underwent ACF for cervical diseases. Informed consent was waived by the Ethics Board of General Hospital of Northern Theater Command because this study was a retrospective, observational study and all of the data were anonymously collected and analyzed.
Medical records of 1,237 patients who underwent ACF at our hospital between January 2013 and December 2019 were identified and reviewed. The inclusion criteria included the following: (1) patients who underwent ACF for cervical diseases and operations performed by one senior surgeon (LX); and (2) hospitalization between January 2013 and December 2019. The exclusion criteria were: (1) patients who underwent surgery for cervical diseases, such as infection, tumor and fracture; (2) patients who also underwent thoracic or lumbar spine surgery or other unrelated procedures; and (3) patients with incomplete data. A total of 1,150 patients were ultimately included in the study (Fig. 1). Patients were categorized into the HT group or no-HT group. The definition of postoperative HT following ACF in the current study was symptomatic postoperative HT manifested as respiratory distress, neurological injury, dysphagia and other symptoms of HT which was confirmed by magnetic resonance imaging (MRI), color Doppler ultrasound or surgical evacuation. Negative pressure drainage devices (Benos Medical Device Co., LTD, Shandong, China) were used in each case in the current study throughout the study period and were generally removed 2–3 days after surgery.
Demographic data, such as age, sex, smoking history, body mass index (BMI), duration of disease, American Society of Anesthesiologists (ASA) physical status (PS) classification, preoperative hemoglobin (Hb), hematocrit, thrombocytocrit, preoperative prothrombin time, international normalized ratio, thrombin time (TT), activated partial thromboplastin time, fibrinogen, and major medical comorbidities, such as diabetes, hypertension, pulmonary, and heart disease (arrhythmia and coronary heart disease), were recorded and analyzed. Anticoagulant therapy meant a history of using low-molecular weight heparin and low-molecular weight heparin should be stopped 24 hours before the operation. Antiplatelet therapy meant a history of taking aspirin and aspirin should be stopped a week before the operation. Preoperative/postoperative systolic/diastolic pressures were recorded. We used computed tomography (CT) to determine the presence of ossification of the posterior longitudinal ligament (OPLL) at the surgical level.
Surgical indicators, such as the number of discs and corpectomy involved, the duration and time of surgery, such as morning (08:00–11:00), noon (11:00–13:00), and afternoon (13:00–), C3 or C7 involvement, intraoperative blood loss (IBL), postoperative drainage (PB), blood transfusion (BT), length of stay, and hospitalization costs, were recorded and analyzed.
The outcome for this study was postoperative complications during hospitalization. The following events were defined as complications: postoperative dyspnoea, cerebrospinal fluid leakage, postoperative HT, poor wound healing, postoperative hypokalemia, postoperative atrial fibrillation, postoperative neurological deterioration, deep venous thrombosis, surgical site infection, pneumonia, urinary tract infection, and bacteremia. All deaths were also considered postoperative complications.
Statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). For counting data, the chi-square test or Fisher exact probability test was used. For measurement data, the Shapiro-Wilk method was used for the normality test, and Student t-test and the Mann-Whitney ranksum test were used for comparisons. We selected the factors which suggested as risk factors for postoperative HT in previous studies and factors such as duration of disease, surgical factors (duration and time of surgery) and C3 or C7 involvement which we thought could have led to the formation of HT. Univariate analysis and multivariate logistic regression analysis was further used to analyze risk factors with a significant difference. A p < 0.05 was regarded to be significantly different.
A total of 1,150 patients with ACF met the inclusion criteria, and 11 patients (1.0%) had postoperative HT. The incidence was 0.9% (10 of 1,150) in postoperative retropharyngeal HTs and 0.1% (1 of 1,150) in spinal epidural HTs. The incidence of AAO caused by a HT was 0.7% (8 of 1,150). HT occurred within 24 hours postoperatively in 5 patients (45.5%), while it occurred at an average of 4 days postoperatively in 6 patients (54.5%). Eight patients (72.7%) underwent HT evacuation, and the other 3 patients underwent conservative treatment, such as close observation, detumescence, and steroids. Among all of the patients, the complications were divided into cerebrospinal fluid leakage (2.5%, 29 of 1,150), postoperative HT (1.0%, 11 of 1,150), poor wound healing (1.4%, 16 of 1,150), neurological deterioration (0.3%, 4 of 1,150), respiratory failure (0.3%, 3 of 1,150), pneumonia (0.2%, 2 of 1,150), atrial fibrillation (0.2%, 2 of 1,150), infection (0.1%, 1 of 1,150) and hypokalemia (0.1%, 1 of 1,150). All patients were successfully treated and discharged (Table 1, Fig. 2).
There were no significant differences in age, BMI, duration of disease, ASA PS classification, trauma history, preoperative coagulation indices, blood pressure, or history of most major medical comorbidities between the 2 groups (Table 2). There were no significant differences in the number of discs and corpectomy involved, duration of operation, time of operation, IBL, PB, BT, C3 involvement, C7 involvement or OPLL at the surgical level (Table 3).
Patients in the HT group presented with a significantly higher frequency of male sex (p = 0.01), smoking history (p = 0.01), and antiplatelet therapy (p = 0.017), a significantly lower preoperative diastolic pressure (p = 0.028) compared with those in the no-HT group (Table 2). Patients with postoperative HT had a higher frequency of complications (p < 0.001), longer days of first-degree/intensive nursing (p < 0.001) and higher hospitalization costs (p = 0.038) (Table 3).
Univariate analysis showed that smoking history (odds ratio [OR], 6.667; 95% confidence interval [CI], 1.434–30.996; p = 0.016), preoperative Hb (OR, 1.046; 95% CI, 1.002–1.093; p = 0.042), preoperative TT (OR, 1.520; 95% CI, 1.0456–2.210; p = 0.028), and antiplatelet therapy (OR, 12.433; 95% CI, 2.524– 61.257; p = 0.002) were risk factors for HT.
Our multivariate logistic regression analysis revealed that smoking history (odds ratio [OR], 5.193; 95% confidence interval [CI], 1.058–25.493; p = 0.042), preoperative TT value (OR, 1.643; 95% CI, 1.104–2.446; p = 0.014), and antiplatelet therapy (OR, 15.070; 95% CI, 2.663–85.274; p = 0.002) remained significant predictors of HT (Table 4).
Postoperative HT after ACF is a rare but potentially catastrophic complication. Some large studies have shown that the incidence of postoperative HT ranges from 0.4% to 5.6% [9-18]. The reported incidence of AAO caused by an HT ranges from 0.2% to 1.9% [18]. In the largest single-surgeon series of ACF operations, we reviewed 1,150 patients and found that 11 patients (1.0%) had postoperative HT, which was consistent with previous reports [9-18]. However, these studies discussed few risk factors for specific patients, such as duration of disease; or surgical factors, such as duration and time of surgery; or C3 or C7 involvement, which could have led to the formation of HTs. In the current study, duration of disease was divided into less than 3 months, 3 months–1 year, 1 year–5 years, and over 5 years, duration of operation was divided into < 150 minutes and ≥ 150 minutes, time of operation was divided into morning (08:00–11:00), noon (11:00–13:00), and afternoon (13:00–), 2 specific cervical spines are mentioned such as C3 and C7.
Risk factors for postoperative HTs included male sex, age older than 65, smoking, higher/lower BMI, anemia, more medical comorbidities, the presence of diffuse idiopathic skeletal hyperostosis, OPLL, therapeutic heparin levels, ASA PS classification grades of III or greater, longer operative times, multiple surgical levels, and increased IBL [11-14,19]. A total of 37,261 anterior cervical discectomy and fusion patients were identified, of whom 148 (0.40%) developed a HT requiring reoperation [11]. Risk factors for the development of HT requiring reoperation were multilevel procedures, a preoperative international normalized ratio > 1.2, lower BMI, ASA PS classification grades of III or greater, preoperative anemia, and male sex [11]. There were no significant differences between the HT group and the no-HT group in the distributions of duration of disease, duration of operation, time of operation, C3 or C7 involvement. Therefore, we speculated that the duration of disease and operation has little influence on the occurrence of postoperative HT. No matter at what time stage (morning, noon, or afternoon) of the operation, the surgeon can complete the operation conscientiously, and does not affect the occurrence of postoperative HT. Different anterior cervical surgery segment, especially C3 or C7 exposure didn’t affect the occurrence of postoperative HT.
In the current study, risk factors for the development of such HTs included smoking history, preoperative TT value and antiplatelet therapy. We routinely assayed the TT as part of a coagulation screen for the preoperative patients. Based on the results obtained from this paper, we recommend preoperative TT measurements for patients undergoing ACF. We found that 18.2% of patients in the HT group and 1.8% of patients in the non-HT group had received preoperative antiplatelet therapy in our study, and antiplatelet therapy was an independent risk factor for HT. Because the p-value of gender was 0.010 by Fisher exact probability test in Table 2, we speculated male gender was enough a risk factor for postoperative HT. We speculated the reason why gender was omitted was because gender did not result in a risk factor for HT by univariate and multivariate analysis by the statical problem due to the zero count cell (female with HT) for a 2× 2 contingency table. These identified risk factors could help surgeons and nurses to plan treatment and case lists. For example, patients at risk for developing HT should be given focused attention to maximize the length of postoperative observation. Postoperative HT in the ACF was associated with a higher frequency of complications, longer days of first-degree/intensive nursing and greater hospitalization costs. These findings are especially important for patient postoperative management and counselling.
It is interesting to discuss the timing of HT in our study. HTs are generally considered to occur mainly within the first few hours after surgery. There were 17 occurrences (0.7%) of postoperative HT in 2,375 ACF procedures. HT occurred within 24 hours postoperatively in 11 patients (65%), while it occurred at an average of 6 days postoperatively in 6 patients (35%) [12]. In a series of AAOs following anterior cervical corpectomy and fusion, 1.15% of patients (9 of 785) developed postoperative HTs. Of these cases, 66.7% (6 of 9) occurred within 24 hours of surgery, while 33.3% (3 of 9) presented at an average of 72 hours postoperatively [20]. A previous study showed that 37% of HTs requiring reoperation occurred after discharge from the hospital [11]. This finding suggests that surgeons should have heightened awareness of this complication throughout the entire 30-day postoperative period and not only during hospitalization [11].
The definition of postoperative HT following ACF in the current study was symptomatic postoperative HT manifested as respiratory distress, neurological injury, dysphagia and other symptoms of HT which was confirmed by MRI, color Doppler ultrasound or surgical evacuation. In the current study, 1 patient with postoperative muscle strength weakened was diagnosed postoperative HT through MRI, 2 patients were diagnosed through color Doppler ultrasound, and other patients underwent emergency exploratory operation and HT evacuation because of respiratory distress. There is a specific type of HT called postoperative delayed HT, which is defined by the progression of neurological deterioration because HTs are observed after more than 3 days. Sokolowski et al. [21] reported a case with symptom onset 13 days after the initial surgery. In the current study, HT occurred within 24 hours postoperatively in 5 patients (45.5%), others occurred at an average of 3 days postoperatively in the 6 patients (54.5%), and 4 patients (36.4%) presented with delayed HT. Although HTs were more common in the acute postoperative period in previous studies, the occurrence of HTs should be considered for at least 2 weeks after surgery.
Drains were used in each case in the current study throughout the study period and were generally removed 2–3 days after surgery. It is important to note that the placement of drains did not prevent postoperative HT [14]. In the current study, drains were in place at the time of HT formation in 63.6% (7 of 11) of patients, and the HT formed shortly (several hours) after drain removal in 9.1% (1 of 11) of patients. Although a HT can form before the drain is removed, it should be noted that removal of the drain could damage vessels or the muscle bed and contribute to HT formation [12]. Therefore, we recommend close monitoring of the patient’s vital signs, especially breathing, for several hours after the drain is removed.
Some researchers have noted that vascular injury and intramuscular bleeding are the main causes of HT [18,22-25]. Incorrect and insufficient hemostasis of the vessels has been identified as one of the main causes of postoperative HT. There have been some reports of dyspnoea being caused by delayed bleeding associated with arterial aneurysms and venous thrombosis [22,26]. In a previous study, surgeons were able to identify a discrete source of bleeding upon secondary surgery in up to 75% of cases in some series [21]. In the current study, the rate was 62.5%, and we found vessel injury in 5 patients (internal opening of the drainage tube in 3 patients and longus cervicalis in 2 patients) upon the secondary operation. We also found that HT could develop without any injury to specific vessels in 3 patients (blood oozing from the lateral wall muscle surface).
Bleeding from exposed cancellous bone after removing ventral osteophytes and dorsal intraspinal canal osteophytes of the spine as well as the site of distractor pin should be taken seriously during operation, we can use bone wax and gelatin sponge to effectively control severe bleeding from exposed cancellous bone during operation. Previous studies have shown that the presence of OPLL was a significant risk factor for HT [10,12,13]. Patients with OPLL usually require extensive osteophyte resection, which can result in greater exposure of cancellous bone, leading to HT formation. Vascular injury occurs during or after surgery and can result in additional bleeding. Therefore, strategies to reduce the incidence of postoperative HT must address multiple potential areas of hemorrhage, including blood vessels, muscles, and exposed cancellous bone. Effective hemostasis and avoidance of excessive intraoperative traction are important for all patients.
The incidence of AAO reported in previous studies ranged from 0.2% to 1.9% [18]. Eight patients (0.7%) presented with AAO caused by HT in the current study. An effective and timely treatment is required to obtain a better therapeutic effect and prognosis of HT-related AAO. The patient’s respiratory status can be assessed by carefully evaluating the condition of the neck and the patient’s behavior [15]. The behavior of patients with controlled dyspnoea or impaired breathing must be closely evaluated. Specifically, spontaneous relief of dyspnoea is impossible when the patient is anxious. Therefore, it is very important to send anxious patients to the operating room immediately to remove the HT, find the cause of bleeding and adopt appropriate hemostatic methods. Patients without fear or anxiety can be treated with oxygen and be closely observed. However, if the patient’s condition deteriorates, the HT should be removed [15,18].
Postoperative respiratory difficulty caused by AAO after ACF is very risky situation because the intratracheal intubation is very difficult. When postoperative respiratory difficulty happens, the first step is to open the incision and relieve the pressure of HT on airway. At the same time, high-flow oxygen inhalation increases the patient's oxygen reserve, to create the conditions for intubation. If the patient cooperates calmly, intubation may be performed while the patient is awake. Under the condition of adequate preparation, the vast majority of patients can be quickly induced and successfully intubated with visual laryngoscope.
According to our experience, postoperative oedema mostly causes eating difficulties and will not cause AAO. Most patients with AAO experience postoperative HT rather than oedema. In the current study, 8 patients presented with swelling of the incision site and respiratory distress, fear and anxiety, so we chose HT evacuation. The preoperative symptoms of all patients resolved immediately or within hours of HT evacuation. Conservative management with steroid treatment was initiated before neurosurgical decompression, resulting in improved neurologic outcomes for patients presenting with mild neurological deterioration [27,28]. We examined 3 cases with conservative treatment, one of which received steroid therapy because his muscle strength weakened temporarily, and it returned to normal approximately 30 minutes later.
Our study has several limitations. First, the retrospective nature of the study can affect the accuracy. Second, factors such as Pack-years for smoking, spinal degeneration, alignment and bone fragility were not discussed in the current study. We will pay much attention to these factors in future prospective studies. Third, the associations between the HT and hemostatic materials were not discussed. We have used bone wax to stop the bleeding from exposed cancellous bone, used gelatin sponge and surgical hemostatic gauze to stop the bleeding from the surface of dura, and all the ACF operations were performed by one senior surgeon (LX), so we were able to rule out the bias caused by the different hemostatic methods and procedures of different surgeons. Forth, the results might have been affected by the relatively limited sample size and nonrandomized design. A multicentre, prospective study with a more detailed assessment of risk factors might be needed.
Smoking history, preoperative TT value and antiplatelet therapy were significant risk factors for postoperative HT following ACF. Postoperative HT in ACF was associated with longer days of first-degree/intensive nursing and greater hospitalization costs. Although HT occurring in the acute postoperative period can be detected and handled in a timely manner, we believe that HT might be a possible cause of airway obstruction even after the acute postoperative period because 54.5% of postoperative HTs occurred in a delayed fashion.
NOTES
Fig. 1.
Flowchart of the study population. ACF, anterior cervical decompression and fusion; HT, hematoma.
Fig. 2.
A 66-year-old man developed postoperative hematoma after C6 anterior cervical corpectomy and fusion (case No. 11). (A, B) Preoperative radiography findings (x-ray and computed tomography) showed a hyperplasic osteophyte at the edge of the vertebral body, which resulted in spinal stenosis. (C-E) Preoperative magnetic resonance imaging (MRI) findings showed spinal stenosis. (F-I) Muscle strength of the patient weakened 2 hours after the operation, emergency MRI was performed. Postoperative MRI findings showed an epidural hematoma, and then muscle strength returned to normal after MRI examination, so the patient received conservative treatment including close observation, detumescence and steroids, and then the patient could walk normally 3 days after operation. (J) Postoperative x-ray showed the good position of internal fixation.
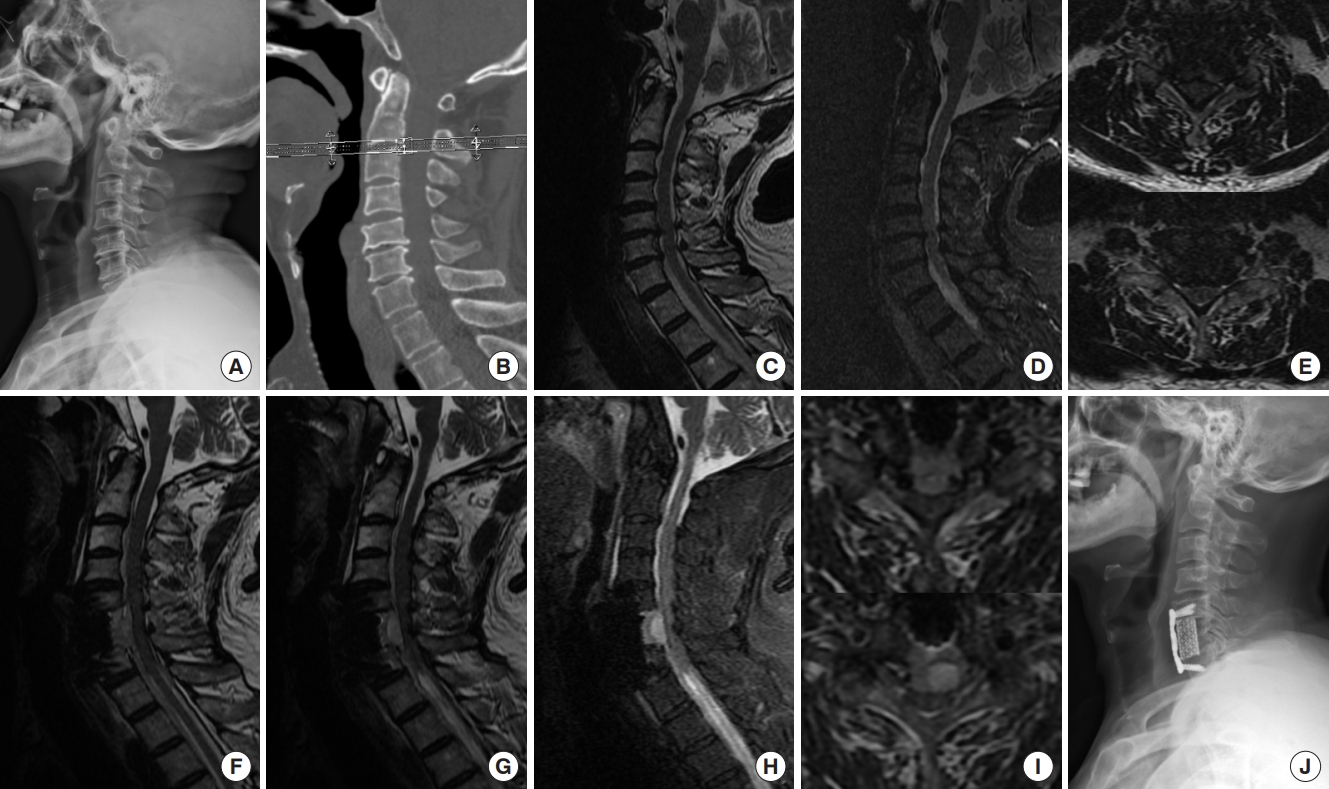
Table 1.
Patients who developed postoperative hematomas
Table 2.
Demographic characteristics of all patients
| Characteristic |
Patients with IBL |
χ2/z | p-value | ||
|---|---|---|---|---|---|
| Hematoma (n = 11) | No hematoma (n = 1,139) | ||||
| Sex | |||||
| Male | 11 | 732 | - | 0.010† | |
| Female | 0 | 407 | |||
| Age (yr) | 51.4 ± 9.5 | 53.8 ± 10.6 | -0.842 | 0.400 | |
| < 60 | 9 (81.8) | 773 (67.9) | - | 0.518† | |
| ≥ 60 | 2 (18.2) | 366 (32.1) | |||
| BMI (kg/m2) | 24.0 ± 2.9 | 24.5 ± 3.4 | |||
| < 25 | 7 (63.6) | 674 (59.2) | |||
| 25–30 | 4 (36.4) | 407 (35.7) | |||
| ≥ 30 | 0 (0) | 58 (5.1) | |||
| Duration of disease | -0.538 | 0.590 | |||
| < 3 Months | 5 (45.5) | 481 (42.2) | - | > 0.999† | |
| 3 Months–1 year | 3 (27.3) | 279 (24.5) | |||
| 1 Year–5 years | 3 (27.3) | 267 (23.4) | |||
| > 5 Years | 0 | 112 (9.8) | |||
| ASA PS classification grade | 1.6 ± 0.7 | 1.5 ± 0.6 | 0.834 | 0.404 | |
| I–II | 10 (90.9) | 1066 (93.6) | - | 0.520† | |
| III–IV | 1 (9.1) | 73 (6.4) | |||
| Trauma history | 1 (9.1) | 292 (25.6) | - | 0.307† | |
| Smoking history | 9 (81.8) | 459 (40.3) | - | 0.010† | |
| Preoperative blood index | |||||
| Hb (g/L) | 149.1 ± 16.3 | 139.7 ± 15.3 | 1.745 | 0.081 | |
| HCT (%) | 41.1 ± 14.3 | 41.6 ± 4.6 | 1.535 | 0.125 | |
| PCT (%) | 0.19 ± 0.05 | 0.18 ± 0.04 | 0.419 | 0.675 | |
| PT (sec) | 13.2 ± 1.0 | 12.6 ± 1.0 | 1.257 | 0.209 | |
| INR | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.819 | 0.413 | |
| TT (sec) | 17.8 ± 1.6 | 17.0 ± 1.3 | 1.953 | 0.051 | |
| APTT (sec) | 34.9 ± 2.6 | 34.8 ± 4.3 | -0.178 | 0.858 | |
| FIB (g/L) | 2.9 ± 0.7 | 3.1 ± 0.8 | -0.684 | 0.494 | |
| Blood pressure | |||||
| Preoperative SP | 127.7 ± 24.8 | 133.9 ± 16.0 | -0.552 | 0.581 | |
| Preoperative DP | 77.3 ± 15.1 | 81.4 ± 9.4 | -2.198 | 0.028 | |
| Postoperative SP | 134.1 ± 21.4 | 129.6 ± 15.8 | 0.651 | 0.515 | |
| Postoperative DP | 77.6 ± 11.4 | 79.4 ± 28.7 | -0.367 | 0.714 | |
| Major medical comorbidities | |||||
| Diabetes | 3 (27.3) | 152 (13.3) | - | 0.176† | |
| Pulmonary disease | 1 (9.1) | 25 (2.2) | - | 0.238† | |
| Hypertension | 3 (27.3) | 235 (20.6) | - | 0.707† | |
| Coronary heart disease | 1 (9.1) | 45 (4.0) | - | 0.363† | |
| Arrhythmia | 1 (9.1) | 88 (7.7) | - | 0.589† | |
| Antiplatelet therapy | 2 (18.2) | 20 (1.8) | - | 0.017† | |
| Anticoagulant therapy | 1 (9.1) | 49 (4.3) | - | 0.388† | |
Values are presented as mean±standard deviation or number (%).
IBL, intraoperative blood loss; BMI, body mass index; ASA PS, American Society of Anesthesiologists physical status; Hb, hemoglobin; HCT, hematocrit; PCT, thrombocytocrit; PT, preoperative prothrombin time; INR, international normalized ratio; TT, thrombin time; APTT, activated partial thromboplastin time; FIB, fibrinogen; SP, systolic pressure; DP, diastolic pressure.
Table 3.
Surgery-related factors, length of stay and hospitalization costs of all the patients
| Characteristic |
Patients with IBL |
χ2/z | p-value | ||
|---|---|---|---|---|---|
| Hematoma (n = 11) | No hematoma (n = 1,139) | ||||
| No. of discs involved | 2.0 ± 0.9 | 1.9 ± 0.8 | 0.521 | 0.602 | |
| 1–2 | 7 (63.6) | 897 (78.8) | - | 0.262† | |
| 3–5 | 4 (36.4) | 242 (21.2) | |||
| Corpectomy | 0.6 ± 0.5 | 0.4 ± 0.5 | 1.688 | 0.091 | |
| 0 | 4 (36.4) | 711 (62.4) | - | 0.115† | |
| 1–2 | 7 (63.6) | 428 (37.6) | |||
| Duration of operation (min) | 123.7 ± 33.5 | 113.0 ± 35.1 | 1.344 | 0.179 | |
| < 150 | 9 (81.8) | 969 (85.1) | - | 0.674† | |
| ≥ 150 | 2 (18.2) | 170 (14.9) | |||
| Time of operation | |||||
| 08:00–11:00 | 7 (63.6) | 752 (66.0) | - | 0.814† | |
| 11:00–13:00 | 1 (9.1) | 162 (14.2) | |||
| 13:00- | 3 (27.3) | 225 (19.8) | |||
| Intraoperative blood loss | 124.5 ± 162.0 | 121.6 ± 155.3 | -0.178 | 0.859 | |
| Postoperative drainage | 107.2 ± 67.7 | 112.0 ± 116.5 | 0.161 | 0.872 | |
| Blood transfusion | 0 (0) | 13 (1.1) | - | > 0.999† | |
| C3 involved | 2 (18.2) | 324 (28.4) | - | 0.738† | |
| C7 involved | 4 (36.4) | 315 (27.7) | - | 0.509† | |
| OPLL among the surgical level | 2 (18.2) | 75 (6.6) | - | 0.165† | |
| Complications | 11 (100) | 53 (4.7) | - | < 0.001† | |
| FN (day) | 5.0 ± 1.9 | 3.4 ± 2.6 | 4.195 | < 0.001 | |
| LOS (day) | 15.5 ± 6.7 | 14.4 ± 4.9 | 0.228 | 0.820 | |
| Cost (*104 RMB) | 8.2 ± 1.6 | 7.2 ± 1.6 | 2.070 | 0.038 | |
Table 4.
Univariate analysis and multivariate logistic regression analysis of the risk factors for hematoma
REFERENCES
1. Wang X, Lin Y, Wang Q, et al. A bibliometric analysis of the top 100 cited articles in anterior cervical discectomy and fusion. J Pain Res 2022;15:3137-56.




2. Deora H, Kim SH, Behari S, et al. Anterior surgical techniques for cervical spondylotic myelopathy: WFNS Spine Committee Recommendations. Neurospine 2019;16:408-20.




3. Park Y, Maeda T, Cho W, et al. Comparison of anterior cervical fusion after two-level discectomy or single-level corpectomy: sagittal alignment, cervical lordosis, graft collapse, and adjacent-level ossification. Spine J 2010;10:193-9.


4. Chen Y, Lü G, Wang B, et al. A comparison of anterior cervical discectomy and fusion (ACDF) using self-locking standalone polyetheretherketone (PEEK) cage with ACDF using cage and plate in the treatment of three-level cervical degenerative spondylopathy: a retrospective study with 2-year follow-up. Eur Spine J 2016;25:2255-62.



5. Jin YZ, Zhao B, Lu XD, et al. Mid- and long-term follow-up efficacy analysis of 3D-printed interbody fusion cages for anterior cervical discectomy and fusion. Orthop Surg 2021;13:1969-78.




6. Bai LL, Wang WT, Wang JF, et al. Anterior cervical discectomy and fusion combined with foraminotomy assisted by high-definition 3-dimensional exoscope in the treatment of cervical spondylotic radiculopathy secondary to bony foraminal stenosis. Orthop Surg 2021;13:2318-26.




7. Sasso RC, Anderson PA, Riew KD, et al. Results of cervical arthroplasty compared with anterior discectomy and fusion: four-year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg Am 2011;93:1684-92.


8. Zhou Q, Zhang J, Liu H, et al. Comparison of anterior and posterior approaches for acute traumatic central spinal cord syndrome with multilevel cervical canal stenosis without cervical fracture or dislocation. Int J Clin Pract 2022;2022:5132134.




9. Epstein N. Frequency, recognition, and management of postoperative hematomas following anterior cervical spine surgery: a review. Surg Neurol Int 2020;11:356.



10. Xia T, Zhou F, Chu H, et al. Incidence and risk factors of postoperative symptomatic spinal epidural hematoma in cervical spine surgery: a single center, retrospective study of 18,220 patients. Eur Spine J 2022;31:2753-60.



11. Bovonratwet P, Fu MC, Tyagi V, et al. Incidence, risk factors, and clinical implications of postoperative hematoma requiring reoperation following anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2019;44:543-9.


12. O’Neill KR, Neuman B, Peters C, et al. Risk factors for postoperative retropharyngeal hematoma after anterior cervical spine surgery. Spine (Phila Pa 1976) 2014;39:E246-52.


13. Miao W, Ma X, Liang D, et al. Treatment of hematomas after anterior cervical spine surgery: a retrospective study of 15 cases. Neurochirurgie 2018;64:166-70.


14. Boudissa M, Lebecque J, Boissière L, et al. Early reintervention after anterior cervical spine surgery: Epidemiology and risk factors: a case-control study. Orthop Traumatol Surg Res 2016;102:485-8.


15. Song KJ, Choi BW, Lee DH, et al. Acute airway obstruction due to postoperative retropharyngeal hematoma after anterior cervical fusion: a retrospective analysis. J Orthop Surg Res 2017;12:19.




16. Debkowska MP, Butterworth JF, Moore JE, et al. Acute postoperative airway complications following anterior cervical spine surgery and the role for cricothyrotomy. J Spine Surg 2019;5:142-54.



17. Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7.


18. Palumbo MA, Aidlen JP, Daniels AH, et al. Airway compromise due to wound hematoma following anterior cervical spine surgery. Open Orthop J 2012;6:108-13.




19. Gennari A, Mazas S, Coudert P, et al. Outpatient anterior cervical discectomy: a French study and literature review. Orthop Traumatol Surg Res 2018;104:581-4.


20. Aono H, Ohwada T, Hosono N, et al. Incidence of postoperative symptomatic epidural hematoma in spinal decompression surgery. J Neurosurg Spine 2011;15:202-5.


21. Sokolowski MJ, Dolan M, Aminian A, et al. Delayed epidural hematoma after spinal surgery: a report of 4 cases. J Spinal Disord Tech 2006;19:603-6.


22. Sethi R, Tandon MS, Ganjoo P. Neck hematoma causing acute airway and hemodynamic compromise after anterior cervical spine surgery. J Neurosurg Anesthesiol 2008;20:69-70.

23. Yi S, Yoon DH, Kim KN, et al. Postoperative spinal epidural hematoma: risk factor and clinical outcome. Yonsei Med J 2006;47:326-32.



24. Liu Z, Jiao Q, Xu J, et al. Spontaneous spinal epidural hematoma: analysis of 23 cases. Surg Neurol 2008;69:253-60. discussion 260.


25. Cullen DJ, Bogdanov E, Htut N. Spinal epidural hematoma occurrence in the absence of known risk factors: a case series. J Clin Anesth 2004;16:376-81.


26. Menon RK, Norris JW. Cervical arterial dissection: current concepts. Ann N Y Acad Sci 2008;1142:200-17.


- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























