 |
 |
- Search
| Neurospine > Volume 21(1); 2024 > Article |
|
|
Abstract
Primary atypical teratoid/rhabdoid tumors (AT/RTs) in the spinal canal are rare central nervous system (CNS) neoplasms that are challenging to diagnose and treat. To date, there has been no standard treatment regimen for these challenging malignant tumors. Thus, we conducted this research to explore potential prognostic factors and feasible treatment modalities for improving the prognosis of these tumors. Articles were retrieved from the PubMed, MEDLINE, and Embase databases, using the keywords “atypical teratoid/rhabdoid tumor,” “rhabdoid tumor,” “spine,” “spinal,” “spinal neoplasm”, and “spinal cord neoplasm.” All eligible cases demonstrated SMARCB1-deficient expression validated by pathological examination. We collected and analyzed data related to clinical presentation, radiological features, pathological characteristics, treatment modalities and prognosis via Kaplan-Meier and Cox regression analyses. Thirty-six articles comprising 58 spinal AT/RT patients were included in the study. The median progression-free survival (PFS) and overall survival (OS) were 18 and 22 months, respectively. Kaplan-Meier analysis demonstrated significant survival improvements for OS in the nonmetastasis, male, radiotherapy and intrathecal chemotherapy groups as well as for PFS in the chemotherapy and radiotherapy groups. Multivariate analysis revealed that chemotherapy and radiotherapy were prognostic factors for improved PFS, and that intrathecal chemotherapy reduced the risk of mortality. Spinal AT/RTs are uncommon malignant entities with a dismal survival rate. Although our review is limited by variability between cases, there is some evidence revealing potential risk factors and the importance of systematic chemotherapy, intrathecal chemotherapy and radiotherapy in spinal AT/RT treatment modalities.
Atypical teratoid/rhabdoid tumors (AT/RTs) are rare and highly aggressive embryonal tumors of the central nervous system (CNS) that were first defined by Rorke et al. in 1996 [1]. Male predominance is observed, and the male to female ratio ranges from 1.3 to 1.5 [2]. Although various ages at diagnosis have been reported from studies, the median age at diagnosis ranges between 6 and 12 months [3,4]. Most cases occur in patients less than 3 years old [3]. While AT/RTs represent 1.6% of all CNS tumors diagnosed in patients less than 19 years old, the percentage has been reported to be 10.1% in patients under 1 year old [3]. AT/RTs predominantly occur in infratentorial and supratentorial locations but rarely in the spinal canal; AT/RTs in the spinal canal account for 2% to 4% of all AT/RTs [3,5]. Biallelic SMARCB1 (INI1, SNF5, BAF47) or rare SMARCA4 (BRG1) inactivation, encoding core subunits of the SWI/SNF chromatin-remodelling complexes, has been shown to be a common genetic alteration and the central event in AT/RTs [2,6]. The five-year overall survival (OS) rates of patients with AT/RTs are between 15% and 50%, even though these rates have recently improved due to multimodal treatment approaches [5]. Older age, radiotherapy, gross total resection (GTR) and absence of metastasis are most consistently related to a better prognosis [7]. Intensive chemotherapy has been validated as an efficient treatment modality for AT/RTs [8].
Due to the uncommon incidence of AT/RTs in the spinal canal, there is no consensus concerning a standard treatment regimen for these tumors, and prognostic factors remain unclear. The aim of this systematic review was to summarize and analyze the demographics and clinical presentations, pathological characteristics, treatment interventions and prognosis of AT/RTs in the spinal canal and to validate potential prognostic factors in patients with this category of tumor.
We retrieved spinal AT/RT-related articles from the PubMed, MEDLINE, and Embase databases. A literature search for publications between 1980 and July 2023 with the following key words was conducted: “atypical teratoid/rhabdoid tumor,” “rhabdoid tumor,” “spine,” “spinal,” “spinal neoplasm”, and “spinal cord neoplasm.”
We included articles adhering to the following criteria: published in English, some or all tumors located in the spinal canal and AT/RTs with SMARCB1-deficient expression validated by pathological examination. Articles were excluded if AT/RT in the spinal canal was not the primary tumor. In all, 598 potentially relevant articles were retrieved. The quality of the included studies was assessed by the Joanna Briggs Institute Checklist for Case Reports and Case series to control the risk of bias. This checklist includes 8 items for case reports and 10 items for case series. The answers for the checklist can be rated as 1 of the 4 options: yes, no, unclear, or not applicable. For case reports, only if the overall scores reached at least 6 points could the studies be included. For case series, overall scores of at least 7 points were required. Two authors assessed the title and abstract of each article and then reviewed full texts meeting the inclusion criteria. A consensus regarding article eligibility was reached according to the inclusion and exclusion criteria. Data regarding clinical presentation, radiological features, pathological characteristics, comprehensive treatment regimens, and prognosis were further analyzed. Progression-free survival (PFS) was defined as the duration from diagnosis to first progression (recurrence or metastasis), death resulting from any cause or last follow-up. OS was defined as the duration from diagnosis until death or last contact. PFS and OS curves were estimated by Kaplan-Meier analysis. The log-rank test and Breslow test were utilized to evaluate the intergroup differences in the single-factor analysis. All parameters with significant differences in the univariate Cox regression analysis were further analyzed in the subsequent multivariate analysis. The hazard ratios and 95% confidence intervals were calculated to validate the independent prognostic factors related to PFS and OS in patients with primary spinal AT/RTs. A p-value of < 0.05 was defined as a significant difference. Statistical analyses were performed with IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA).
To identify potential prognostic factors for spinal AT/RTs, we explored the PubMed, MEDLINE, and Embase databases using relevant keywords. After the exclusion of 562 articles, 36 articles met the eligibility criteria and described in detail 58 patients with primary spinal AT/RTs from 1980 to July 2023 that were validated by pathological examination (Fig. 1) [7,9-43]. Spinal AT/RT tumors in 7 cases (12.3%) invaded and broke through the dura or leptomeninges. Most patients (30 cases, 52.6%) had intradural and extramedullary tumors, and 20 patients (35.1%) and 14 patients (24.6%) had intramedullary and extradural tumors, respectively. Eleven cases (19.0%) exhibited extension into the intervertebral foramen, and 8 cases (13.8%) demonstrated infiltration of nerve roots on neuroimaging. The male to female ratio was close to 1 (29:28), and the median age at diagnosis was 4 years (range, 0.3–65.0 years). The majority of the tumors were located in the lumbar and thoracic segments (35 cases, 60.3%; 32 cases, 55.2%, respectively), and the rest were located in the cervical and sacral segments (19 cases, 32.8%; 12 cases, 20.7%, respectively). Among the 58 cases, 31 cases (53.4%) showed involvement of more than one spinal segment. The most frequently reported clinical presentations were pain and extremity weakness, with a median clinical history duration of 1 month (range, 0.2–29.5 months) in 33 patients based on the longest symptomatic duration of each of these patients. The median maximum diameter of the tumors in 19 patients was 5.8 cm (range, 1.4–14.3 cm), with 8 tumors (61.5%) and 5 tumors (38.5%) showing obscure and clear boundaries, respectively. The presentation of T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) signals was highly variable; nevertheless, 36 tumors (62.1%) had enhancement, and heterogeneous enhancement was more common in 14 of these tumors (38.9%). There were 14 cases (25.0%) of leptomeningeal dissemination at preoperative diagnosis and 12 cases (21.4%) of leptomeningeal dissemination at postoperative diagnosis. Moreover, 1 case (1.8%) of leptomeningeal dissemination was confirmed by autopsy. Apart from the overlapping cases, 23 cases (41.1%) of leptomeningeal dissemination were found. Moreover, 8 patients developed hydrocephalus during the clinical course. The demographic and clinical features of these patients are summarized in Table 1.
Forty-eight patients underwent surgical treatment: subtotal resection (STR) in 31 patients (64.6%) and GTR in 17 patients (35.4%). Nine patients were treated with secondary operations after disease progression. Additionally, 30 patients were treated with postoperative radiotherapy, and 39 patients were treated with postoperative chemotherapy. Fourteen of 39 patients (35.9%) treated with chemotherapy underwent intrathecal chemotherapy.
Immunohistochemical examinations indicated that vimentin was positive in 24 of all patients examined for vimentin, S100 in 3 of 10, desmin in 1 of 16, glial fibrillary acidic protein in 3 of 19, epithelial membrane antigen in 33 of 36, cytokeratin in 24 of 33, anti-smooth muscle antibody in 11 of 17, neurofilament protein in 6 of 7, CD99 in 6 of 12 and synaptophysin in 4 of 14. The loss of SMARCB1 protein was validated by immunohistochemical examinations in all cases, and the index of Ki-67 labelling ranged from 10% to 90% in 14 cases. Furthermore, tumors in 14 cases (24.1%) demonstrated haemorrhage and necrosis. The treatment modalities and pathological characteristics of these patients are summarized in Table 2.
The mean follow-up duration was 18.1 months, with a range of 0.6–93.0 months. Nine patients suffered from recurrence (9 of 33, 27.3%), and 19 patients developed metastasis (19 of 37, 51.4%), including 6 cases (6 of 19, 31.6%) with intracranial metastasis and 1 case with extra-axial metastasis. Twenty-eight patients passed away during the follow-up period (28 of 51, 54.9%). The 2-year PFS and OS rates were 39.5% and 42.8%, respectively. The follow-up and prognosis of these patients are summarized in Table 3.
A summary of the descriptive statistical data is shown in Table 4. The median PFS and OS were 18 and 22 months, respectively, as demonstrated by the Kaplan-Meier curves in Fig. 2A and B, respectively. Patients who received radiotherapy (log-rank test: p < 0.001; Breslow test: p < 0.001) or intrathecal chemotherapy (log-rank test: p = 0.003; Breslow test: p = 0.003) had a longer OS than those who did not receive radiotherapy or intrathecal chemotherapy according to Kaplan-Meier analysis (Fig. 2C and D, respectively). In addition, a significant difference in OS for the metastasis (log-rank test: p = 0.002; Breslow test: p = 0.020) and sex (log-rank test: p = 0.040; Breslow test: p = 0.056) groups was observed based on Kaplan-Meier analysis (Fig. 2E and F, respectively); this finding indicated that patients without metastasis and males had a better OS than those with metastasis and females, respectively. A similar analysis indicated that patients receiving chemotherapy (log-rank test: p = 0.001; Breslow test: p = 0.001) or radiotherapy (log-rank test: p = 0.007; Breslow test: p = 0.006) exhibited a better PFS (Fig. 2G and H, respectively). Multivariate Cox regression analysis revealed that patients treated with chemotherapy (p = 0.010) or radiotherapy (p = 0.034) had a lower risk for disease progression than those without chemotherapy or radiotherapy. A homologous analysis found a lower mortality risk for patients treated with intrathecal chemotherapy (p = 0.042). The statistical analysis results of these patients are summarized in Table 5.
AT/RTs are highly invasive and aggressive CNS embryonal tumors that are most frequently located in supratentorial and infratentorial locations and rarely in the spinal canal [3]. AT/RTs occur in 1.6% of patients under 19 years old diagnosed with CNS tumors, and spinal AT/RT cases account for 2%–4% of validated AT/RT cases [3,5]. AT/RTs mainly affect children younger than 3 years with a slight male predominance. AT/RTs in adults are rare; nonetheless, accumulated cases have demonstrated that AT/RTs in the sellar region exclusively arise in adult females, implicating biological differences from other AT/RTs [44]. A study comprising 6 cases of sellar AT/RTs by Nakata et al. [44] demonstrated an age range from 21 to 69 years and a significant female predominance in which all patients were female. However, there was no significant sex predominance detected in our research. Nonetheless, a significant difference was observed in the sex group, which indicated that males had a better prognosis than females. The median age of the 44 patients in our study was 4 years, with a range of 0.3–65.0 years, which was generally consistent with prior research. Younger patients at the time of diagnosis of AT/RTs have been identified as having a worse prognosis than older patients [5,45,46]. Although there is a tendency for improved survival in patients 4 to 17 years old compared with patients aged under 1 to 2 years at diagnosis, our research did not reveal a significant difference in survival based on age [47].
While GTR could improve the prognosis of patients with AT/RTs, it is often difficult to perform GTR at primary surgery due to the large size and abundant vascularity of spinal AT/RTs [46]. Previous studies indicated that GTR is achieved in 30%–50% of AT/RTs [8,46,47]. The GTR rate in our study was merely up to 35.4%. To boost the GTR rate of spinal AT/RTs, neoadjuvant chemotherapy is necessary, which may diminish not only the size but also the vascularity of tumors and facilitate subsequent resection [48]. Unfortunately, no case in our research was observed to utilize neoadjuvant therapy. Even though the utilization of neoadjuvant therapy in AT/RTs is infrequent, a study of a small cohort conducted by Ishisaka et al. [48] in which ICE or modified IRS-III regimens were administered to patients preoperatively was noted and indicated that neoadjuvant therapy could diminish the volume and vascularity of tumors, but no improvement in prognosis was observed.
Due to a lack of large amounts of clinical data on primary spinal AT/RTs, there are currently no effective and mature treatment regimens. An aggressive surgical approach is the mainstay of treatment for spinal AT/RTs. A study by Hilden et al. [46] indicated that GTR could improve the PFS of patients and the prognosis of AT/RTs. GTR was achieved in 35.4% of patients in our research. Maximal-degree resection of the primary tumor has been associated with improved PFS and OS [47]. Significant differences in OS and PFS for the administration of surgery or the extent of tumor resection were not observed based on Kaplan-Meier analysis. Nevertheless, patients who underwent surgery had longer median OS (24 months vs. 7 months) and PFS (20 months vs. 14 months) than did those who did not undergo surgery, and patients who underwent GTR had longer median OS (42 months vs. 15 months) and PFS (20 months vs. 11 months) than did those who underwent STR. Therefore, an aggressive surgical approach is recommended to achieve GTR, which could alleviate symptoms, sufficiently reduce tumor volume, obtain tumor specimens for pathological examinations and form the basis for subsequent treatment. En bloc tumor resection with negative margins is often regarded as an effective method of achieving long-term PFS or OS in some cases of malignant spinal neoplasms [49]. Only one case in our study was treated with en bloc resection and there were no reports to be found regarding the extent of surgical margins. Because of the malignant biological behavior of spinal AT/RTs, the tumors commonly invade nerve roots, vertebral arteries and cervical spinal cord. Obtaining a negative surgical margin is difficult because damage to the aforementioned structures during aggressive tumor resection will cause severe complications and even death. Nonetheless, pursuing a goal of negative surgical margins would benefit the patients greatly under the premises of extensive presurgical planning, multidisciplinary surgical teams with rich experience managing these tumors and early-stage input of rehabilitation physicians following aggressive resections [50]. Surgical-related complications depend on both aggressive surgical resection and the patient’s medical background. We did not observe any early (less than 30 days postoperatively) complications in our study but observed one patient with chronic (more than 30 days postoperatively) complications and progressive thoracolumbar kyphosis 5 years after emergent T9-L3 laminectomies for STR. Twenty-three patients in our study did not develop neurological deficits or deterioration of vital status in the early postoperative stage. Given the malignant biological behavior of these tumors, after extensive discussion with the patients and their respective families, surgery should not be compromised to avoid neurological deficits with the aim of saving life when the tumors, such as those located in the cervical segments, endanger the lives of patients. Conversely, if the tumors cannot endanger the patient’s life shortly, surgery should be performed with a view to avoiding the development of neurological deficits and providing the patient with optimum long-term tumor control. In 23 available cases with disease progression, we observed 9 patients undergoing secondary surgery. However, there were no significant differences demonstrating that secondary surgery could improve the prognosis of patients with disease progression. Considering spinal AT/RTs as systemic diseases and highly malignant tumors, multiple operations could increase the risks of drop metastasis and severe neurological deficits and even cause the death of patients. Meanwhile, chemotherapy and radiotherapy have shown progress in helping to improve the prognosis of spinal AT/RTs. Thus, it might be more prudent to choose secondary surgery treatment when the patient suffers a relapse.
Of the immunohistochemical markers, Ki-67 antibody has been validated as providing the most valuable approach for evaluating the cell proliferation extent of CNS neoplasms [51]. Ki-67 was recorded in 14 cases, ranging from 10% to 90%. No significant difference was observed in our study probability due to the limited Ki-67 data. Thus, efforts to obtain more clinical data are warranted in the future. SMARCB1 has been validated as a genuine tumor suppressor gene in genetically engineered mouse models; biallelic inactivation of SMARCB1 located in chromosome band 22q11 [2]. is the most ubiquitous genetic alteration in AT/RTs [2,52]. SMARCB1 inactivation has a variety of causes such as deletions, loss of heterozygosity and mutations, which result in the mutation of subunits of SWI/SNF complexes encoded by the SMARCB1 gene, the central propulsion event in the development of AT/RTs [2].
Our analysis demonstrated that the utilization of chemotherapy and intrathecal chemotherapy could be potential positive prognostic factors in spinal AT/RTs, as validated by Kaplan-Meier analysis and multivariate Cox regression analysis. The patients receiving intrathecal chemotherapy had improved OS and a lower mortality risk, which might be associated with a high incidence of leptomeningeal dissemination of spinal AT/RTs. High-dose chemotherapy (HDC) combined with stem cell rescue (SCR) is considered an effective regimen for AT/RT and allows radiotherapy to be deferred in very young children to prevent neurocognitive deficits [53]. The Head Start and CCG99703 regimens represented the first generation of such studies, from which there were reports of favorable prognosis for a proportion of patients with HDC/SCR. Slavc et al. [54] reported a series with an improved 5-year OS of 100% in 9 patients undergoing an intensive multimodal regimen consisting of a dose-dense regimen combined with intrathecal chemotherapy followed by HDC/SCR. Importantly, as the largest prospective research for AT/RTs, the ACNS0333 trial produced results that confirmed HDC/SCR as an important regimen in AT/RTs.
In terms of therapeutic regimens for more frequent embryonal CNS tumors, radiotherapy is deemed to represent an important regimen for spinal AT/RTs. While the utilization of radiotherapy in spinal AT/RT patients remains controversial due to severe neurocognitive side effects in young children, Buscariollo et al. [47] found that younger age had an even stronger relationship with a more favorable prognosis when radiotherapy was utilized as the initial treatment. In one case reported by Yano et al. [33] in our research, it was noted that a patient, treated with GTR and intensive systematic and intrathecal chemotherapy with autologous bone marrow transplantation upon diagnosis at 1.75 years of age, received radiotherapy to the local tumor bed and craniospinal axis after 1 year and maintained complete remission without neurological deficits at the last follow-up at 4 years of age. Decision-making regarding the benefit-risk of radiotherapy use for treating children with AT/RTs remains difficult. Nevertheless, such cases highlight that dynamic and individual radiotherapy designs might be necessary for some children in the early-stage of spinal AT/RTs. Conversely, for very young patients with relapse, the survival profit may outweigh the possible hazard, and focal irradiation of small volumes can be performed to minimize side effects [55]. Stereotactic radiosurgery (SRS) is a potential therapeutic option compared with conventional radiation therapies in that SRS enables a highly conformal and large dose radiation to be delivered to a localized tumor tissue while minimizing radiation exposure in normal tissue. The safety of SRS for treating patients with spinal tumors has been confirmed by multiple reports [56-59]. A study by Benzil et al. [57] revealed that SRS was safe with minimal risk of complications and provided effective pain relief in the treatment of spinal tumors, but did not indicate obvious improvement of prognosis. Another study by Shin et al. [56] demonstrated effectiveness in tumor control and the improvement of neurological function for spinal metastases. Nonetheless, the long-term survival of patients undergoing SRS is still dismal. Spina et al. [58] conducted a systematic review regarding SRS for the management of AT/RTs and found that local tumor control was achieved in 66.7% (8 of 12) of cases, among which 4 cases were alive with a mean survival time of 61.3 months. SRS was administered in one patient with intracranial metastasis postoperatively, and the patient died 2.5 years after diagnosis in our study. Despite the lack of long-term survival improvement evidence, potential benefits of utilizing SRS in the management of spinal AT/RTs as an initial irradiation regimen are avoiding the increased risks of radiotherapy-related neurocognitive and endocrine toxicity, alleviating symptoms and improving local tumor control for young children with spinal AT/RTs. These relationships among age, irradiation utilization, and magnitude of the relevant difference in survival for spinal AT/RTs warrant validation in clinical trials. Compared with historical therapies, the ACNS0333 regimen conducted by Reddy et al. [8], utilizing high-dose methotrexate induction with HDC/SCR consolidation combined with involved-field and age-adapted timing radiotherapy, dramatically improved the survival of patients with AT/RTs and might provide a promising reference for spinal AT/RT treatment.
Of the 50 patients included in our analysis, the median OS was 22 months, as calculated by Kaplan-Meier analysis, which was longer than the range of 6 to 18 months in previously published studies on AT/RTs [47,60]. Spinal AT/RTs in our research revealed that the characteristics of surrounding invasion and metastasis on neuroimaging were ubiquitous, including multiple spinal-segment involvement, invasion and breaking through leptomeninges or dura, extension into interverbal foramens, infiltration of nerve roots and presentation of obscure boundaries at diagnosis. The frequency of tumor metastasis was 51.4% in 37 patients with available information, and this value was higher than the range (i.e., 20% to 40%) reported in prior studies regarding AT/RTs [4]. Furthermore, 6 patients developed intracranial metastasis, and one patient developed extra-axial metastasis. Our research indicated that metastasis was associated with poorer OS. A higher incidence of metastasis and poorer survival of patients with spinal AT/RTs indicated that tumors in this category were more aggressive and invasive than cranial AT/RTs. Approximately 25%–35% of AT/RT patients with germline mutations in SMARCB1 are diagnosed in generally younger patients than those without SMARCB1 germline mutations, who show a predisposition to concurrent tumors and more extensive disease [2,8]. Thus, patients, particularly at an earlier age, should undergo whole-body examinations to detect potential extra-axial lesions, if pathological examination validates the diagnosis of spinal AT/RTs. Postoperative neuroimaging examination including the whole neural axis is warranted to find early-stage cranial and spinal metastasis and perform timely intervention in spinal AT/RT patients. Moreover, we assessed 14 cases showing signs of leptomeningeal dissemination on neuroimaging at preoperative diagnosis and 12 cases showing leptomeningeal dissemination after operation administration. Additionally, one case of leptomeningeal dissemination was confirmed by autopsy. Apart from the overlapping cases, 23 cases of leptomeningeal dissemination were found. Leptomeningeal dissemination accounts for one-third of AT/RTs, and its prognosis is poor, with a median survival of 1–4 months [61,62]. A study by Calandrelli et al. [62] described 4 patients with leptomeningeal dissemination, accounting for 40% of all pediatric patients at diagnosis. Another study on AT/RTs by Dardis et al. [63] demonstrated that 12 cases of leptomeningeal dissemination accounted for 24% of 50 cases and that adults with leptomeningeal dissemination had a grave prognosis. While survival analysis indicated that leptomeningeal dissemination was not a prognostic factor for OS in our research, our review of clinical findings indicated that spinal AT/RTs adjacent to cerebrospinal fluid (CSF) resulted in a high incidence of leptomeningeal dissemination and the presence of metastasis. Therefore, the early-stage detection of leptomeningeal dissemination in spinal AT/RTs should be highlighted for its high incidence to guide treatment regimens and evaluate prognosis. Moreover, 8 cases were identified as hydrocephalus during the clinical course. Despite no significant differences between the hydrocephalus and nonhydrocephalus groups, the median OS of patients with hydrocephalus was shorter than that of patients with nonhydrocephalus (16 months in 7 patients vs. 30 months in 43 patients). Neoplastic leptomeningeal dissemination, extension of tumors in high-level spinal segments and intracranial metastasis to block CSF pathways might be mechanisms for the development of hydrocephalus in spinal AT/RTs [64].
This review is limited by undetailed and incomplete data as well as small sample size. Because of the importance of cell proliferation, more information regarding Ki-67 should be collected and analyzed to assist clinicians in evaluating the prognosis of spinal AT/RTs. More regimens such as neoadjuvant therapy, SRS and secondary surgery should be applied in a large-scale study to further validate their efficacy. Owing to the lack of DNA methylation profiling and gene expression profiling information, we could not classify spinal AT/RTs into 3 molecular subtypes according to the fifth edition of the World Health Organization classification of CNS tumors. Molecular analysis, such as whole-genome or whole-exome sequencing, should be conducted in more cases of spinal AT/RTs. Structural variants, mutations and DNA methylation profiling should be screened, collected, and analyzed in the future to explore pathogenesis and potential targeted agents against spinal AT/RTs.
Primary spinal AT/RTs are aggressive malignant tumors with a dismal patient survival rate, even after intensive treatment regimens. Metastasis and female sex were associated with poorer prognosis. As the basis of spinal AT/RT therapeutic regimens, surgical resection, particularly maximal-degree resection, could prolong the median OS and PFS of patients. Chemotherapy might defer the time of disease progression and reduce the risk of disease progression. We also found that intrathecal chemotherapy could extend the OS of patients. Radiotherapy was validated as a feasible and effective therapeutic regimen that could prolong the OS and PFS of patients and lower the risk of disease progression. However, further larger-scale prospective studies of spinal AT/RTs are warranted to confirm these findings and explore additional prognostic factors and specific treatment regimens.
NOTES
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. AT/RT, atypical teratoid/rhabdoid tumor.
Fig. 2.
Kaplan-Meier curves of PFS (A) and OS (B). Patients who received radiotherapy (C) or intrathecal chemotherapy (D) had a longer OS. A significant difference in OS for the metastasis (E) and sex (F) groups was observed. Better PFS values were observed in the groups of patients receiving chemotherapy (G) or radiotherapy (H). PFS, progression-free survival; OS, overall survival.
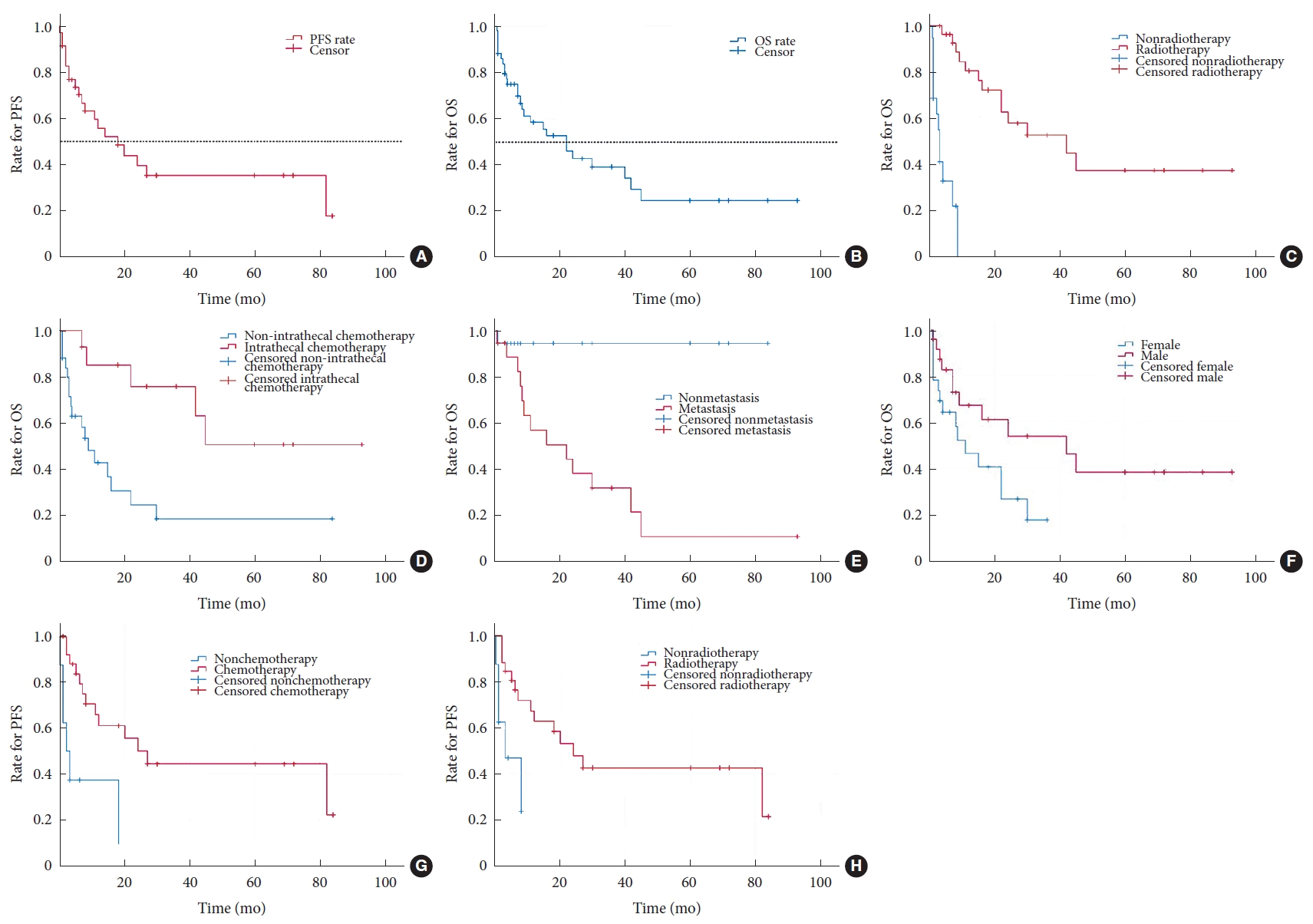
Table 1.
The demographic and clinical features of these patients
| No. | Study | Level of evidence | Cohort size | Location | Sex | Age (yr) | Clinical symptom | Duration of symptom | Maximum diameter | Boundary | T1WI | T2WI | Enhancement | Hydrocephalus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zarovnaya et al. [34]/2007 | V | 1 | C4 to C6 (intradural and extramedullary; intramedullary) | F | 43.0 | Neck pain and left UE pain | 1 Week | NA | Obscure | NA | NA | NA | No |
| 2 | Bannykh et al. [10]/2006 | V | 1 | T9 to L1 (intradural and extramedullary; extradural); extended bilateral neural foramina | M | 4.0 | Bilateral LE weakness; lower back pain; right hip pain; constipation; loss of sensation below the L1 | A few days | NA | NA | NA | NA | Heterogeneous enhancement | No |
| 3 | Benesch et al. [7]/2020 | IV | 13 | Lumbar (n = 10), thoracic (n = 10), sacral (n = 3), and cervical (n = 2). (intramedullary) | M (5/13) | NA | Bladder and/or bowel dysfunction (n = 6), pain (n = 6), motor and/or sensory deficits (n = 5), raised intracranial pressure (n = 1), gait disturbances (n = 1), and decreased mobility (n = 1) | 1 Day-8 weeks; median: 2 weeks | NA | NA | NA | NA | NA | No |
| F (8/13) | ||||||||||||||
| 4 | Sinha et al. [29]/2015 | V | 1 | T12 (intradural and extramedullary); infiltrating the nerve roots of the left side | M | 65.0 | Bilateral LE weakness; bilateral LE pain | 5 Weeks | NA | Clear | Iso | Hypo | Homogeneous enhancement | Yes |
| Painless urinary retention | 1 Day | |||||||||||||
| Perianal numbness | NA | |||||||||||||
| 5 | Gotti et al. [16]/2015 | V | 1 | L4 to L5 (intradural and extramedullary) | F | 19.0 | Back pain | 1 Month | NA | NA | NA | NA | Homogeneous enhancement | No |
| 6 | Dhir et al. [14]/2015 | V | 1 | Below L3 (intradural and extramedullary); thickening of the adjacent cauda-equina nerve roots | M | 2.5 | Inability to ambulate; urinary/fecal incontinence | 29.5 Months | 7 cm | Clear | NA | NA | Minimally enhancement | No |
| 7 | Babgi et al. [9]/2018 | V | 1 | T12 to L1 (intramedullary; intradural and extramedullary); dural thickening; multiple nodular enhancement along the spinal cord and the surface of the brain stem | M | 6.0 | Fall; back pain | 1 Week | NA | NA | Hypo to iso | Heterogeneous hypo | Heterogeneous enhancement | Yes |
| 8 | Kramer et al. [21]/2020 | V | 1 | L1 to L2 and conus medullaris (intradural and extramedullary) | F | 5.0 | Bilateral LE pain | NA | 3 cm | NA | Hypo | Hypo | Mild heterogeneous enhancement | No |
| 9 | Niwa et al. [26]/2009 | V | 1 | C4 to C6 (intradural and extramedullary); extended left neural foramina | M | 6.0 | Left UE pain and left cervical pain | 3 Months | NA | Obscure | Iso | Iso | Moderate enhancement | No |
| 10 | Kelley et al. [20]/2012 | V | 1 | T10 to L1 (intradural and extramedullary) | M | 4.0 | Constipation | 2 Weeks | NA | NA | NA | NA | NA | No |
| Bilateral LE weakness | Several days | |||||||||||||
| 11 | Shiflett et al. [27]/2020 | V | 1 | T11 to L5 (intramedullary); thickening of the nerve roots; leptomeningeal enhancement | F | 1.4 | Bilateral LE paralysis/weakness and decreased rectal tone | NA | NA | NA | Hyper | Heterogenous hyper | Heterogenous enhancement | No |
| 12 | Garling et al. [15]/2018 | V | 1 | L3 to S3 (intradural and extramedullary) and extending to the left S1–S3 neural foramina to the sacral plexus and presacral region | F | 15.0 | Bilateral LE weakness; bilateral LE numbness; constipation and decreased sensation in bilateral LE | 3 Months | 14 cm | NA | NA | NA | NA | No |
| Urinary/fecal incontinence | 1 Week | |||||||||||||
| 13 | Singla et al. [28]/2016 | V | 1 | T12 to S1 (extradural); T6 (syrinx) | M | 0.3 | Left LE weakness | NA | NA | Clear | Iso | Iso | NA | No |
| 14 | Moeller et al. [25]/2007 | V | 1 | T11 to L2 (intradural and extramedullary) | M | 9.0 | Right LE numbness; bilateral LE weakness/paralysis; urinary incontinence | 3 Months | NA | NA | Hypo | Iso | Diffuse enhancement | No |
| 15 | Yano et al. [33]/2008 | V | 1 | C2 to C6 (intradural and extramedullary; extradural); enlargement of the right C4 to C5 intervertebral foramen | F | 1.8 | Bilateral severe UE weakness/paresis; Bilateral moderate LE weakness/paresis | 1 Week | NA | NA | Hyper | Heterogeneous | Heterogenous enhancement | No |
| 16 | Heuer et al. [18]/2010 | V | 1 | Clivus to C2 (extradural and bony erosion) | M | 7.0 | Neck pain | 11 Months | NA | NA | NA | NA | Heterogenous enhancement | No |
| Photophobia | 4 Months | |||||||||||||
| 17 | Xin et al. [31]/2014 | V | 1 | C2 to C5 (extradural); invasion of C3 vertebral body, left vertebral plate, left transverse foramen, and bilateral neural foramens | F | 10.0 | Nape pain | 2 Months | NA | NA | Hypo | Hyper | NA | No |
| 18 | Yang et al. [32]/2007 | V | 1 | L2 to L4 (intradural and extramedullary); some small spots over the lumbar region | M | 7.0 | Back pain and unstable gait | 3 Weeks | 4 cm | Obscure | NA | NA | Heterogenous enhancement | No |
| 19 | Hong and Ogiwara [19]/2018 | V | 1 | C3 to C5 (intradural and extramedullary; extradural) | M | 3.0 | Right UE weakness to quadriparesis; urinary/fecal incontinence | 1 Week | NA | NA | NA | NA | Heterogenous enhancement | No |
| 20 | Hale et al. [17]/2017 | V | 1 | T8 (intradural and extramedullary); multiple nodules involved with conus medullaris and distal lumbosacral nerve roots | F | 4.0 | Bilateral LE pain and lower back pain | 2 Weeks | 1.4 cm | Obscure | NA | Hypo | Heterogenous enhancement | No |
| 21 | Tsitsopoulos et al. [30]/2020 | V | 1 | C4 to T1 (extradural); left C6 to C7 and C7 to T1 foramen; thoracic cavity | M | 0.3 | Hypotonia; poor head control; reduced level of consciousness | 1 Week | NA | NA | NA | NA | Heterogenous enhancement | Yes |
| 22 | Mankotia et al. [23]/2016 | V | 1 | T5 to T10 (intradural and extramedullary); pontomedullary junction up to T8 (associated syrinx) | M | 5.0 | Quadriparesis; respiratory distress; sensory loss below the level of nipples | 1 Week | NA | Obscure | Heterogeneous hyper | Heterogeneous hypo | Heterogenous enhancement | No |
| 23 | McGinity et al. [24]/2017 | V | 1 | C1 to C3 (intradural and extramedullary) | F | 43.0 | Neck pain and headache | NA | NA | NA | NA | NA | NA | No |
| 24 | Chao et al. [13]/2017 | V | 1 | T11 to L3 (intradural and extramedullary) | M | 1.3 | Urinary retention | 1 Month | 6.9 cm | NA | NA | Iso | Heterogenous enhancement | No |
| Bilateral LE weakness | 1 Week | |||||||||||||
| 25 | Buccoliero et al. [12]/2019 | IV | 1/2 | L1 to L5 (intradural extramedullary; extradural; extraspinal extension) | M | 1.5 | Left LE pain and left LE weakness | NA | NA | NA | NA | NA | NA | No |
| 2/2 | T11 to L2 (intramedullary) | F | 3.7 | Difficulty walking; pelvis pain and bilateral LE pain | NA | NA | NA | NA | NA | NA | No | |||
| 26 | Li et al. [22]/2019 | IV | 1/3 | C2 to C6 (intramedullary); exophytic component at C4 | F | 2.3 | Neck pain | 1 Month | NA | NA | NA | NA | Heterogenous enhancement | Yes |
| 2/3 | T12 to L1 (intradural and extramedullary) | F | 5.3 | Bilateral LE pain | NA | NA | NA | NA | Hypo | Mild heterogenous enhancement | Yes | |||
| 3/3 | C5 to T1 (intradural and extramedullary); C7, T1, left paraspinal muscles and brachial plexus (extradural) | M | 11.3 | Bilateral UE weakness and back pain | NA | NA | NA | NA | NA | Heterogenous enhancement | No | |||
| 27 | Biswas et al. [11]/2023 | V | 1 | C3 to T1 (extradural); C6 to C7 and right paraspinal region (extradural) | F | 1.5 | Neck deviation | 4 Months | 4.3 cm | Clear | Iso | Hyper | Enhancement | No |
| Quadriparesis | 20 Days | |||||||||||||
| 28 | Meena et al. [38]/2020 | V | 1 | C3 to C7 (intradural and extramedullary); C5 to C6 (extradural); diffuse leptomeningeal enhancement | M | 3.0 | Flaccid quadriplegia | 12 Days | NA | Obscure | Hyper | Hypo | Mild heterogeneous enhancement | No |
| Loss sensation below C4 level | NA | |||||||||||||
| Respiratory arrest and pain | NA | |||||||||||||
| 29 | Mohapatra et al. [39]/2010 | IV | 1 | C1 to C2 | M | 4.5 | NA | NA | NA | NA | Hypo | Iso | Heterogenous enhancement | No |
| 30 | Yang et al. [42]/2014 | IV | 1 | C2 to T2 (intramedullary) | F | 0.7 | Progressive limbs weakness | NA | 2 cm | Obscure | Hyper | Iso to hyper | Heterogenous enhancement | No |
| 31 | Neromyliotis et al. [40]/2019 | V | 1 | L4 to S2 (intradural and extramedullary) | F | 19.0 | Coccydynia and unsteady gait | 3 Months | 7 cm | NA | Iso | Hypo | Heterogenous enhancement | No |
| 32 | Wu et al. [41]/2018 | IV | 1/8 | T10 to L1 (intradural and extramedullary); sacral canal; spinal meningesenhancement and nerve root enhancement | F | 3.0 | Headache | 2 Months | 5.8 cm | NA | Iso | Slightly hyper | Slight enhancement | Yes |
| Bilateral LE pain and weakness | 20 Days | |||||||||||||
| 2/8 | Medulla oblongata, T6 to T8, T11 to L1, L5 and sacral canal (intradural and extramedullary); intervertebral foramen invasion, intracranial and spinal meningeal enhancement | F | 3.0 | Headache and vomiting | 3 Months | 2.1 cm | NA | Slightly hyper | Slightly hyper | Obvious enhancement | Yes | |||
| Bilateral LE weakness | 3 Days | |||||||||||||
| 3/8 | T6 to T12 (extradural); spinal meninges and nerve root enhancement | F | 3.0 | Asthenia of the lower limbs | 1 Week | 10.1 cm | NA | Slightly hyper | Slightly hyper | Slight enhancement | No | |||
| Urinary retention | 2 Days | |||||||||||||
| 4/8 | L3 to S1 (intradural and extramedullary); sacral canal, spinal meninges and nerve root enhancement | F | 5.0 | Bilateral LE pain | 15 Days | 2.8 cm | NA | Slightly hyper | Slightly hyper | Obvious enhancement | No | |||
| 5/8 | T9 to L3 (intradural and extramedullary); spinal meninges enhancement; nerve root enhancement | M | 2.0 | Bilateral LE asthenia | 8 Days | 11.5 cm | NA | Iso | Slightly hyper | Obvious enhancement | Yes | |||
| 6/8 | L1 to L3 (extradural); enlargement of intervertebral foramen; paravertebral soft tissue shadow and spinal meninges enhancement | M | 3.0 | Right LE pain | 1 Month | 6 cm | NA | Iso | Slightly hyper | Obvious enhancement | No | |||
| 7/8 | Below T9 and sacral canal (intradural and extramedullary); enlargement of intervertebral foramen; paravertebral soft tissue shadow and spinal meninges enhancement | M | 2.0 | Bilateral lower limbs asthenia | 5 Days | 14.3 cm | NA | Iso | Slightly hyper | Slight enhancement | No | |||
| Urinary retention | 1 Day | |||||||||||||
| 8/8 | Below L3, sacral canal (extradural); sacrococcygeal region (paravertebral); sacral destruction | F | 3.0 | Bilateral LE weakness; incontinence | 30 Days | 9.1 cm | NA | Iso | Slightly hyper | Heterogenous enhancement | No | |||
| 33 | Amit et al. [35]/2020 | V | 1 | C5 to C6 (intradural and extramedullary); right brachial plexus (paraspinal); erosion of the posterior wall of the foramen transversarium | M | 14.0 | Right neck and shoulder pain | 3 Months | 2.4 cm | Clear | Hypo | Hyper | Homogeneous enhancement | No |
| Right hemi paresis | 2 Days | |||||||||||||
| 34 | Biswas et al. [36]/2021 | V | 1 | T10 to T12 (intradural and extramedullary) | M | 6.0 | Low back pain; bilateral LE weakness | 5 Months | 4.7 cm | NA | Iso | Iso | NA | No |
| Loss of sensation below the umbilicus; constipation and urinary retention | 2 Weeks | |||||||||||||
| 35 | Broggi et al. [37]/2022 | IV | 1 | L4 to L5 (intramedullary) | NA | NA | Back pain and leg paresthesia | NA | NA | Obscure | NA | NA | Homogeneous enhancement | No |
| 36 | Wolfe et al. [43]/2018 | V | 1 | C2 to C4 (extradural); left C2 to C3 neural foramen widening | M | 13.0 | Left neck pain | 10 Months | NA | NA | NA | NA | Enhancement | No |
Table 2.
The treatment modalities and pathological characteristics of these patients
| No. | Study | Cohort size |
Immunohistochemistry pathology |
Extent of resection | Secondary surgery | Postoperative chemotherapy | Postoperative radiotherapy | Intrathecal chemotherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vimentin | S100 | Desmin | GFAP | EMA | CK | SMA | Neurofilament protein | CD99 | Synaptophysin | Ki-67 | ||||||||
| 1 | Zarovnaya et al. [34]/2007 | 1 | NA | NA | NA | - | + | - | NA | NA | NA | - | 30% | STR | Yes | Yes | Yes (SRS) | No |
| 2 | Bannykh et al. [10]/2006 | 1 | + | NA | NA | - | + | + | + | NA | NA | NA | 47% | NA | NA | Yes | Yes | Yes |
| 3 | Benesch et al. [7]/2020 | 1/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | STR | NA | Yes | No | Yes |
| 2/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | GTR | NA | Yes | Yes | Yes | ||
| 3/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | STR | NA | Yes | Yes | Yes | ||
| 4/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | STR | No | Yes | No | No | ||
| 5/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | STR | No | Yes | Yes | Yes | ||
| 6/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Yes | Yes | No | ||
| 7/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Yes | No | No | ||
| 8/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | STR | NA | Yes | Yes | No | ||
| 9/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | STR | NA | Yes | No | No | ||
| 10/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Yes | No | No | ||
| 11/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | STR | NA | Yes | No | No | ||
| 12/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | GTR | NA | Yes | No | No | ||
| 13/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | STR | No | Yes | Yes | No | ||
| 4 | Sinha et al. [29]/2015 | 1 | + | - | - | NA | + | - | - | NA | NA | - | NA | STR | Yes | No | Yes | NA |
| 5 | Gotti et al. [16]/2015 | 1 | + | NA | NA | NA | + | + | NA | NA | + | NA | NA | STR | Yes | Yes | Yes | Yes |
| 6 | Dhir et al. [14]/2015 | 1 | NA | - | - | - | NA | + | NA | NA | - | + | NA | GTR | NA | Yes | No | No |
| 7 | Babgi et al. [9]/2018 | 1 | NA | NA | NA | - | + | NA | NA | NA | NA | - | 90% | STR | Yes | Yes | Yes | No |
| 8 | Kramer et al. [21]/2020 | 1 | + | - | NA | - | + | - | NA | + | - | - | 50% | GTR | NA | Yes | Yes | Yes |
| 9 | Niwa et al. [26]/2009 | 1 | + | NA | - | NA | NA | + | + | NA | NA | NA | NA | STR | NA | NA | NA | NA |
| 10 | Kelley et al. [20]/2012 | 1 | + | NA | NA | NA | + | + | + | NA | NA | NA | 47% | STR | NA | Yes | Yes | Yes |
| 11 | Shiflett et al. [27]/2020 | 1 | NA | NA | + | + | NA | NA | NA | NA | NA | NA | NA | GTR | No | No | No | NA |
| 12 | Garling et al. [15]/2018 | 1 | + | NA | NA | NA | NA | - | NA | NA | - | NA | NA | STR | NA | Yes | No | No |
| 13 | Singla et al. [28]/2016 | 1 | + | NA | - | - | - | - | - | NA | + | - | 15%–30% | GTR | No | No | No | NA |
| 14 | Moeller et al. [25]/2007 | 1 | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | NA | GTR | NA | No | Yes | NA |
| 15 | Yano et al. [33]/2008 | 1 | NA | NA | NA | - | + | + | NA | NA | NA | NA | 21.2% | GTR | NA | Yes | Yes | Yes |
| 16 | Heuer et al. [18]/2010 | 1 | NA | NA | NA | NA | + | + | NA | NA | NA | NA | NA | GTR (en bloc resection) | Yes | Yes | Yes | Yes |
| 17 | Xin et al. [31]/2014 | 1 | + | NA | NA | NA | + | + | NA | NA | + | + | NA | STR | No | Yes | Yes | No |
| 18 | Yang et al. [32]/2007 | 1 | + | - | - | - | + | + | - | + | + | - | NA | NA | No | Yes | Yes | No |
| 19 | Hong and Ogiwara [19]/2018 | 1 | + | NA | NA | NA | + | + | + | NA | NA | NA | NA | STR | NA | Yes | Yes | No |
| 20 | Hale et al. [17]/2017 | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | No | No | NA |
| 21 | Tsitsopoulos et al. [30]/2020 | 1 | NA | - | - | NA | - | + | NA | NA | NA | NA | NA | NA | No | Yes | Yes | No |
| 22 | Mankotia et al. [23]/2016 | 1 | + | NA | - | - | + | + | - | NA | NA | NA | 30% | STR | NA | Yes | No | No |
| 23 | McGinity et al. [24]/2017 | 1 | + | NA | NA | NA | + | NA | + | NA | NA | NA | NA | GTR | NA | No | Yes | NA |
| 24 | Chao et al. [13]/2017 | 1 | + | NA | - | - | + | NA | - | NA | NA | - | NA | GTR | Yes | Yes | Yes | Yes |
| 25 | Buccoliero et al. [12]/2019 | 1/2 | NA | - | - | - | + | - | NA | + | NA | NA | 80% | STR | NA | Yes | No | No |
| 2/2 | NA | + | - | - | + | + | NA | + | NA | NA | 10% | STR | NA | Yes | No | No | ||
| 26 | Li et al. [22]/2019 | 1/3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | GTR | Yes | Yes | No | Yes |
| 2/3 | + | - | NA | - | + | - | NA | + | - | - | 50% | GTR | Yes | Yes | Yes | Yes | ||
| 3/3 | NA | + | - | + | + | NA | + | NA | NA | NA | NA | STR | Yes | Yes | Yes | Yes | ||
| 27 | Biswas et al. [11]/2023 | 1 | + | NA | NA | NA | + | NA | + | NA | + | NA | NA | STR | NA | Yes | Yes | No |
| 28 | Meena et al. [38]/2020 | 1 | NA | NA | - | - | + | NA | + | NA | NA | + | NA | STR | NA | No | No | NA |
| 29 | Mohapatra et al. [39]/2010 | 1 | + | NA | - | + | + | - | - | - | NA | - | 30% | NA | NA | NA | NA | NA |
| 30 | Yang et al. [42]/2014 | 1 | + | + | - | - | + | + | + | NA | - | - | NA | STR | NA | No | No | NA |
| 31 | Neromyliotis et al. [40]/2019 | 1 | + | NA | NA | NA | + | + | NA | + | NA | + | 40% | STR | No | No | Yes | NA |
| 32 | Wu et al. [41]/2018 | 1/8 | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | NA | STR | NA | No | No | NA |
| 2/8 | + | NA | NA | NA | + | + | NA | NA | NA | NA | NA | STR | No | No | No | NA | ||
| 3/8 | + | NA | NA | NA | + | + | NA | NA | NA | NA | NA | GTR | NA | No | No | NA | ||
| 4/8 | + | NA | NA | NA | + | + | NA | NA | NA | NA | NA | GTR | NA | No | No | NA | ||
| 5/8 | NA | NA | NA | NA | + | + | NA | NA | NA | NA | NA | STR | No | No | No | NA | ||
| 6/8 | NA | NA | NA | NA | + | - | NA | NA | NA | NA | NA | GTR | No | Yes | Yes | No | ||
| 7/8 | NA | NA | NA | NA | - | + | NA | NA | NA | NA | NA | STR | NA | No | No | NA | ||
| 8/8 | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | No | No | NA | ||
| 33 | Amit et al. [35]/2020 | 1 | NA | NA | NA | NA | + | + | NA | NA | + | NA | NA | STR | No | Yes | Yes | No |
| 34 | Biswas et al. [36]/2021 | 1 | NA | NA | - | - | + | + | + | NA | - | NA | 35%–40% | GTR | NA | Yes | Yes | No |
| 35 | Broggi et al. [37]/2022 | 1 | NA | NA | NA | NA | NA | + | + | NA | NA | NA | NA | NA | No | NA | NA | NA |
| 36 | Wolfe et al. [43]/2018 | 1 | + | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | STR | NA | Yes | Yes | No |
Table 3.
The follow-up and prognosis of these patients
| No. | Study | Cohort size | Follow-up time (mo) | Postoperative complications | Recurrence | Metastasis | Outcome | PFS (mo) | OS (mo) | Leptomeningeal dissemination | Survival from leptomeningeal dissemination to death (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 | Yang et al. [32]/2007 | 1 | 7.0 | NA | Yes | Yes | Died | 5.0 | 7.0 | Yes (pre/postoperative) | 2 |
| 19 | Hong et al. [19]/2018 | 1 | 5.0 | No | No | No | Alive | 5.0 | 5.0 | No | NA |
| 20 | Hale et al. [17]/2017 | 1 | 0.6 | NA | NA | NA | Died | NA | NA | Yes | NA |
| 21 | Tsitsopoulos et al. [30]/2020 | 1 | 3.8 | NA | NA | Yes (intracranial) | Died | NA | 3.8 | No | NA |
| 22 | Mankotia et al. [23]/2016 | 1 | 1.0 | No | No | No | Alive | 1.0 | 1.0 | No | NA |
| 23 | McGinity et al. [24]/2017 | 1 | 6.0 | No | No | No | Alive | 6.0 | 6.0 | No | NA |
| 24 | Chao et al. [13]/2017 | 1 | 7.0 | No | Yes | No | Alive | 3.0 | 7.0 | No | NA |
| 25 | Buccoliero et al. [12]/2019 | 1/2 | 3.0 | NA | NA | NA | Died | NA | 3.0 | No | NA |
| 2/2 | 4.0 | NA | NA | NA | Died | NA | 4.0 | No | NA | ||
| 26 | Li et al. [22]/2019 | 1/3 | 8.5 | NA | Yes | Yes | Died | 8.0 | 8.5 | Yes (postoperative) | 0.5 |
| 2/3 | 22.0 | NA | No | Yes (intracranial) | Died | 20.0 | 22.0 | Yes (postoperative) | 2 | ||
| 3/3 | 45.0 | NA | No | Yes (intracranial) | Died | 24.0 | 45.0 | Yes (postoperative) | 11 | ||
| 27 | Biswas et al. [11]/2023 | 1 | 30.0 | NA | No | No | Alive | 30.0 | 30.0 | No | NA |
| 28 | Meena et al. [38]/2020 | 1 | 1.0 | No | NA | NA | Died | NA | 1.0 | Yes (preoperative) | 1 |
| 29 | Mohapatra et al. [39]/2010 | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 30 | Yang et al. [42]/2014 | 1 | 1.0 | NA | NA | No | Died | NA | 1.0 | No | NA |
| 31 | Neromyliotis et al. [40]/2019 | 1 | 3.0 | No | Yes | Yes | Alive | 2.0 | 3.0 | Yes (postoperative) | NA |
| 32 | Wu et al. [41]/2018 | 1/8 | NA | NA | NA | NA | NA | NA | NA | Yes (preoperative) | NA |
| 2/8 | 3.0 | NA | No | Yes | Alive | 3.0 | 3.0 | Yes (preoperative) | NA | ||
| 3/8 | NA | NA | NA | NA | NA | NA | NA | Yes (preoperative) | NA | ||
| 4/8 | NA | NA | NA | NA | NA | NA | NA | Yes (preoperative) | NA | ||
| 5/8 | 1.0 | NA | Yes | Yes (intracranial) | Alive | 1.0 | 1.0 | Yes (pre/postoperative) | NA | ||
| 6/8 | 12.0 | NA | Yes | No | Alive | 12.0 | 12.0 | Yes (preoperative) | NA | ||
| 7/8 | NA | NA | NA | NA | NA | NA | NA | Yes (preoperative) | NA | ||
| 8/8 | NA | NA | NA | NA | NA | NA | NA | No | NA | ||
| 33 | Amit et al. [35]/2020 | 1 | 9.0 | No | No | Yes | Died | 6.0 | 9.0 | No | NA |
| 34 | Biswas et al. [36]/2021 | 1 | 30.0 | No | No | Yes | Alive | 30.0 | 30.0 | Yes (postoperative) | NA |
| 35 | Broggi et al. [37]/2022 | 1 | 40.0 | NA | NA | NA | Died | 14.0 | 40.0 | No | NA |
| 36 | Wolfe et al. [43]/2018 | 1 | 84.0 | NA | No | No | Alive | 84.0 | 84.0 | No | NA |
Table 4.
The summary of clinical characteristics and prognostic information
Table 5.
The results of the log-rank test, Breslow test and univariate and multivariate Cox regression analysis
REFERENCES
1. Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg 1996;85:56-65.


2. Frühwald MC, Biegel JA, Bourdeaut F, et al. Atypical teratoid/rhabdoid tumors-current concepts, advances in biology, and potential future therapies. Neuro Oncol 2016;18:764-78.



3. Ostrom QT, Chen Y, M de Blank P, et al. The descriptive epidemiology of atypical teratoid/rhabdoid tumors in the United States, 2001-2010. Neuro Oncol 2014;16:1392-9.



4. Fossey M, Li H, Afzal S, et al. Atypical teratoid rhabdoid tumor in the first year of life: the Canadian ATRT registry experience and review of the literature. J Neurooncol 2017;132:155-62.



5. Frühwald MC, Hasselblatt M, Nemes K, et al. Age and DNA methylation subgroup as potential independent risk factors for treatment stratification in children with atypical teratoid/rhabdoid tumors. Neuro Oncol 2020;22:1006-17.




6. Mittal P, Roberts CWM. The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat Rev Clin Oncol 2020;17:435-48.




7. Benesch M, Nemes K, Neumayer P, et al. Spinal cord atypical teratoid/rhabdoid tumors in children: clinical, genetic, and outcome characteristics in a representative European cohort. Pediatr Blood Cancer 2020;67:e28022.



8. Reddy AT, Strother DR, Judkins AR, et al. Efficacy of high-dose chemotherapy and three-dimensional conformal radiation for atypical teratoid/rhabdoid tumor: a report from the Children’s Oncology Group Trial ACNS0333. J Clin Oncol 2020;38:1175-85.



9. Babgi M, Samkari A, Al-Mehdar A, et al. Atypical teratoid/rhabdoid tumor of the spinal cord in a child: case report and comprehensive review of the literature. Pediatr Neurosurg 2018;53:254-62.



10. Bannykh S, Duncan C, Ogle E, et al. Atypical teratoid/rhabdoid tumor of the spinal canal. J Neurooncol 2006;76:129-30.



11. Biswas A, Ghosh V, Roy S, et al. Spinal atypical teratoid rhabdoid tumor-narrative review and report of a rare case managed with multimodality approach. Childs Nerv Syst 2023;39:2019-26.



12. Buccoliero AM, Caporalini C, Scagnet M, et al. A diagnostic pitfall: atypical teratoid rhabdoid tumor versus dedifferentiated/poorly differentiated chordoma: analysis of a mono-institutional series. Appl Immunohistochem Mol Morphol 2019;27:147-54.


13. Chao MF, Su YF, Jaw TS, et al. Atypical teratoid/rhabdoid tumor of lumbar spine in a toddler child. Spinal Cord Ser Cases 2017;3:16026.




14. Dhir A, Tekautz T, Recinos V, et al. Lumbar spinal atypical teratoid rhabdoid tumor. J Clin Neurosci 2015;22:1988-9.


15. Garling RJ, Singh R, Harris C, et al. Intradural lumbosacral malignant extrarenal rhabdoid tumor: a case report. Childs Nerv Syst 2018;34:165-7.



16. Gotti G, Biassoni V, Schiavello E, et al. A case of relapsing spinal atypical teratoid/rhabdoid tumor (AT/RT) responding to vinorelbine, cyclophosphamide, and celecoxib. Childs Nerv Syst 2015;31:1621-3.



17. Hale AT, Fricker GP, Crook TW. A case of a 4-year-old female with a primary spinal malignancy presenting with Froin’s syndrome. Pediatr Neurosurg 2017;53:64-8.



18. Heuer GG, Kiefer H, Judkins AR, et al. Surgical treatment of a clival-C2 atypical teratoid/rhabdoid tumor. J Neurosurg Pediatr 2010;5:75-9.



19. Hong S, Ogiwara H. Dumbbell-shaped atypical teratoid rhabdoid tumor in the cervical spine mimicking schwannoma. Childs Nerv Syst 2018;34:27-8.



20. Kelley BJ, Johnson MH, Vortmeyer AO, et al. Two-level thoracic pedicle subtraction osteotomy for progressive postlaminectomy kyphotic deformity following resection of an unusual thoracolumbar intradural extramedullary tumor. J Neurosurg Pediatr 2012;10:334-9.


21. Kramer DE, Kerolus MG, Nunna RS, et al. Atypical teratoid/rhabdoid tumor of the conus medullaris. Pediatr Neurosurg 2020;55:215-21.



22. Li D, Heiferman DM, Syed HR, et al. Pediatric primary spinal atypical teratoid rhabdoid tumor: a case series and review of the literature. J Neurosurg Pediatr 2019 Jul 12:1-17. doi:10.3171/2019.4.PEDS19113. [Epub].
23. Mankotia DS, Tandon V, Sharma BS, et al. A case of primary spinal atypical teratoid/rhabdoid tumor in a 5-year-old child. J Pediatr Neurosci 2016;11:121-4.



24. McGinity M, Siddiqui H, Singh G, et al. Primary atypical teratoid rhabdoid tumor in the adult spine. Surg Neurol Int 2017;8:34.



25. Moeller KK, Coventry S, Jernigan S, et al. Atypical teratoid/rhabdoid tumor of the spine. AJNR Am J Neuroradiol 2007;28:593-5.


26. Niwa T, Aida N, Tanaka M, et al. Diffusion-weighted imaging of an atypical teratoid/rhabdoid tumor of the cervical spine. Magn Reson Med Sci 2009;8:135-8.


27. Shiflett JM, Herrington BL, Joyner DA, et al. Atypical teratoid rhabdoid tumor of the cauda equina in a child: report of a very unusual case. Appl Immunohistochem Mol Morphol 2020;28:e58-62.


28. Singla N, Kapoor A, Chatterjee D, et al. Ultra early recurrence in giant congenital malignant rhabdoid tumor of spine. Childs Nerv Syst 2016;32:2471-4.



29. Sinha P, Ahmad M, Varghese A, et al. Atypical teratoid rhabdoid tumor of the spine: report of a case and literature review. Eur Spine J 2015;24 Suppl 4:S472-84.

30. Tsitsopoulos PP, Marinos K, Chochliourou E, et al. Infantile atypical teratoid rhabdoid tumor of the spine presenting with acute hydrocephalus. Pediatr Neurosurg 2020;55:313-8.



31. Xin X, Zhu B, Shen J, et al. A primary spinal extradural atypical teratoid/rhabdoid tumor of the cervical spine with bony involvement. J Child Neurol 2014;29:670-3.



32. Yang CS, Jan YJ, Wang J, et al. Spinal atypical teratoid/rhabdoid tumor in a 7-year-old boy. Neuropathology 2007;27:139-44.


33. Yano S, Hida K, Kobayashi H, et al. Successful multimodal therapies for a primary atypical teratoid/rhabdoid tumor in the cervical spine. Pediatr Neurosurg 2008;44:406-13.



34. Zarovnaya EL, Pallatroni HF, Hug EB, et al. Atypical teratoid/rhabdoid tumor of the spine in an adult: case report and review of the literature. J Neurooncol 2007;84:49-55.



35. Amit A, Vats A, Shoakazemi A, et al. Dumbell atypical teratoid/rhabdoid tumor (AT/RT) of the cervical spine. British J Neurosurg 2020;34:339-41.
36. Biswas A, Velu U, Sharma S, et al. Successful multimodality management of atypical teratoid/rhabdoid tumor of the lower dorsal spine with spinal drop metastasis: illustrated review. Pediatr Neurosurg 2021;56:184-96.



37. Broggi G, Gianno F, Shemy DT, et al. Atypical teratoid/rhabdoid tumor in adults: a systematic review of the literature with meta-analysis and additional reports of 4 cases. J Neurooncol 2022;157:1-14.



38. Meena RK, Doddamani RS, Chipde H, et al. Primary spinal atypical teratoid/rhabdoid tumor presenting with hematomyelia and subarachnoid haemorrhage-a case report. Childs Nerv Syst 2020;36:655-9.



39. Mohapatra I, Santosh V, Chickabasaviah YT, et al. Histological and immunohistochemical characterization of AT/RT: a report of 15 cases from India. Neuropathology 2010;30:251-9.


40. Neromyliotis E, Kalyvas AV, Drosos E, et al. Spinal atypical rhabdoid teratoid tumor in an adult woman: case report and review of the literature. World Neurosurg 2019;128:196-9.


41. Wu HY, Xu WB, Lu LW, et al. Imaging features of spinal atypical teratoid rhabdoid tumors in children. Medicine 2018;97:e13808.



42. Yang M, Chen X, Wang N, et al. Primary atypical teratoid/rhabdoid tumor of central nervous system in children: a clinicopathological analysis and review of literature in China. Int J Clin Exp Pathol 2014;7:2411-20.


43. Wolfe AD, Capitini CM, Salamat SM, et al. Neck rhabdoid tumors: clinical features and consideration of autologous stem cell transplant. J Pediatr Hematol Oncol 2018;40:e50-4.



44. Nakata S, Nobusawa S, Hirose T, et al. Sellar atypical teratoid/rhabdoid tumor (AT/RT): a clinicopathologically and genetically distinct variant of AT/RT. Am J Surg Pathol 2017;41:932-40.

45. Dufour C, Beaugrand A, Le Deley MC, et al. Clinicopathologic prognostic factors in childhood atypical teratoid and rhabdoid tumor of the central nervous system: a multicenter study. Cancer 2012;118:3812-21.


46. Hilden JM, Meerbaum S, Burger P, et al. Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol 2004;22:2877-84.


47. Buscariollo DL, Park HS, Roberts KB, et al. Survival outcomes in atypical teratoid rhabdoid tumor for patients undergoing radiotherapy in a Surveillance, Epidemiology, and End Results analysis. Cancer 2012;118:4212-9.


48. Ishisaka E, Usami K, Kiyotani C, et al. Neoadjuvant chemotherapy for atypical teratoid rhabdoid tumors (AT/RTs). Childs Nerv Syst 2020;36:721-7.



49. Fourney DR, Rhines LD, Hentschel SJ, et al. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine 2005;3:111-22.


50. Akinduro OO, Garcia DP, Domingo RA, et al. Cervical chordomas: multicenter case series and meta-analysis. J Neurooncol 2021;153:65-77.



51. Prayson RA. The utility of MIB-1/Ki-67 immunostaining in the evaluation of central nervous system neoplasms. Adv Anat Pathol 2005;12:144-8.


52. Roberts CW, Galusha SA, McMenamin ME, et al. Haploinsufficiency of Snf5 (integrase interactor 1) predisposes to malignant rhabdoid tumors in mice. Proc Natl Acad Sci U S A 2000;97:13796-800.



53. Hoffman LM, Richardson EA, Ho B, et al. Advancing biology-based therapeutic approaches for atypical teratoid rhabdoid tumors. Neuro Oncol 2020;22:944-54.




54. Slavc I, Chocholous M, Leiss U, et al. Atypical teratoid rhabdoid tumor: improved long-term survival with an intensive multimodal therapy and delayed radiotherapy. The Medical University of Vienna Experience 1992-2012. Cancer Med 2014;3:91-100.




55. Pai Panandiker AS, Merchant TE, Beltran C, et al. Sequencing of local therapy affects the pattern of treatment failure and survival in children with atypical teratoid rhabdoid tumors of the central nervous system. Int J Radiat Oncol Biol Phys 2012;82:1756-63.



56. Shin DA, Huh R, Chung SS, et al. Stereotactic spine radiosurgery for intradural and intramedullary metastasis. Neurosurg Focus 2009;27:E10.



57. Benzil DL, Saboori M, Mogilner AY, et al. Safety and efficacy of stereotactic radiosurgery for tumors of the spine. J Neurosurg 2004;101 Suppl 3:413-8.


58. Spina A, Gagliardi F, Boari N, et al. Does stereotactic radiosurgery positively impact the local control of atypical teratoid rhabdoid tumors? World Neurosurg 2017;104:612-8.


59. Detsky JS, Nguyen TK, Lee Y, et al. Mature imaging-based outcomes supporting local control for complex reirradiation salvage spine stereotactic body radiotherapy. Neurosurgery 2020;87:816-22.



60. Chi SN, Zimmerman MA, Yao X, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol 2009;27:385-9.


61. Lamba N, Wen PY, Aizer AA. Epidemiology of brain metastases and leptomeningeal disease. Neuro Oncol 2021;23:1447-56.




62. Calandrelli R, Massimi L, Pilato F, et al. Atypical teratoid rhabdoid tumor: proposal of a diagnostic pathway based on clinical features and neuroimaging findings. Diagnostics (Basel) 2023;13:475.































