- Search
|
|
||
See commentary "Commentary on “Navigation-Guided/Robot-Assisted Spinal Surgery: A Review Article”" in Volume 21 on page 18.
Abstract
The development of minimally invasive spinal surgery utilizing navigation and robotics has significantly improved the feasibility, accuracy, and efficiency of this surgery. In particular, these methods provide improved accuracy of pedicle screw placement, reduced radiation exposure, and shortened learning curves for surgeons. However, research on the clinical outcomes and cost-effectiveness of navigation and robot-assisted spinal surgery is still in its infancy. Therefore, there is limited available evidence and this makes it difficult to draw definitive conclusions regarding the long-term benefits of these technologies. In this review article, we provide a summary of the current navigation and robotic spinal surgery systems. We concluded that despite the progress that has been made in recent years, and the clear advantages these methods can provide in terms of clinical outcomes and shortened learning curves, cost-effectiveness remains an issue. Therefore, future studies are required to consider training costs, variable initial expenses, maintenance and service fees, and operating costs of these advanced platforms so that they are feasible for implementation in standard clinical practice.
In recent years, spinal surgery has undergone substantial advancements, with significant developments in surgical equipment, operative techniques, implants, and biological enhancements. The integration of computer-assisted navigation and robotic systems has marked a pivotal evolution in this field.
One breakthrough in this domain is the development of systems that process and reconstruct data from 3-dimensional (3D) spinal maps, providing real-time image guidance and navigation. Initially, these systems used radiographic imaging and stereotaxy to enhance precision and accuracy during surgery. In 1908, Horsley and Clarke [1] first attempted lesion targeting using a stereotactic frame with the Wonsoon brain, paving the way for the development of frameless stereotaxy coupled with real-time imaging/navigation in cranial surgery by 1990 [2,3].
The emergence of robotic surgical systems has significantly influenced spinal surgery. These systems are predominantly employed for screw insertion, whereby radiographic imaging is used to follow the surgeon’s desired screw trajectory with an automated robotic arm [4]. The U.S. Food and Drug Administration (FDA)-approved da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) has been influential in the field as it is used in various surgical areas because of its immersive 3D system, contributing to the popularization of surgical robotics [5].
Current robotic systems in spinal surgery can be categorized into 3 types [6]: First, supervisory controlled systems: here, the surgeon plans the surgery offline, and the robot autonomously performs the procedure under the supervision of the surgeon. Second, telesurgical systems: the surgeon controls the robot remotely in real time. Third, shared-control systems: both the surgeon and robot directly control the surgical instruments simultaneously.
Currently, spinal surgery predominantly uses shared-control systems that determine the stereotactic trajectory using preoperative and intraoperative imaging. However, navigation systems provide enhanced real-time stereotactic feedback to the surgeon, who uses this information to manually perform the surgery.
The use of navigation has grown, with increasing evidence supporting its accuracy in thoracolumbar pedicle screw placement. The advantages of using image-guided navigation and robotic systems for spinal surgery are multifaceted. First, these systems offer improved accuracy compared with traditional freehand pedicle screw insertion techniques, reduced radiation exposure compared with fluoroscopic-assisted techniques; further, they enable minimally invasive applications. Second, robotic systems can overcome human limitations by improving the precision of tasks that may be affected by fatigue, tremors, and repetitiveness.
In this review article, we provide a summary of the current navigation and robotic spinal surgery systems, their advantages and disadvantages, associated clinical outcomes, and their current and potential future clinical applications.
The da Vinci Surgical System was approved by the FDA for laparoscopic surgery in 2000. The utilization of this system has gradually increased, and it is currently applied in cardiac, thoracic, and urological surgeries. This system provides × 10 magnification to the operator and 3D vision of the surgical field. It also provides tremor control and a wide angle of motion [5]. The da Vinci Surgical System is used for laparoscopic anterior lumbar interbody fusion during spinal surgery [7]. However, although this system is useful for complex and minimally invasive intracavitary surgical procedures, research on its applications in spinal surgery, especially pedicle screw placement, is lacking.
Various computer-assistant navigation (CAN) systems have been used in the spinal surgery field. The currently available 3D CAN platforms include Airo Mobile Intraoperative computer tomography-based Spinal Navigation (Brainlab, Feldkirchen, Germany), Stryker Spinal Navigation with SpineMask Tracker and SpinalMap Software (Stryker, Kalamazoo, MI, USA), Stealth Strarion, Spine Surgery Imaging and Surgical Navigation with O-arm (Medtronic, Minneapolis, MN, USA), and Ziehm VisionFD Vario 3D with NaviPort integration (Ziehm Imaging, Orlando, FL, USA).
Typically, both the Stealth Station with O-arm and the Ziehm Vision FD Vario 3D provide real-time, 3D surgical images. The O-arm features a 360° scanner, whereas the Ziehm Vision FD Vario 3D utilizes a 190° rotational scan around the patient. The images are then reformatted to create a 360° 3D map. Instrument registration and real-time navigation are employed simultaneously. Consequently, 3D images of the spine can be displayed on the screen during surgery, eliminating the need to adjust the patient’s position.
The use of CAN in spinal surgery has been increasing, primarily owing to its accuracy and safety in pedicle screw placement. In a meta-analysis, Verma et al. [8] evaluated 5,992 pedicle screws and reported a high accuracy rate of pedicle screw placement with the use of CAN. Furthermore, Shin et al. [9] compared the incidence of pedicle screw breach between CAN and the free-hand technique, reporting rates of 6% and 15%, respectively. This finding suggests that CAN increases the accuracy of pedicle screw placement. Conversely, Gelalis et al. [10] compared the free-hand technique, fluoroscopy, and CAN, and reported no significant difference in accuracy between fluoroscopy and CAN. In addition, the application of CAN has expanded to include treatment of spinal column and intradural tumors, infections, and deformities, as well as revision spinal surgeries [11].
SpineAssist (Mazor Robotics Ltd., Carsarea, Israel) was the first robotic system to receive FDA approval in 2004, marking a pivotal moment in the integration of robotics into spinal surgery. In 2011, the second-generation robotic system, Renaissance, was developed as a replication of SpineAssist with notable improvements including a smaller, lighter design, thus providing enhanced imaging [12].
In 2016, Mazor Robotics Ltd. released the third-generation Mazor X system. A significant innovation in the Mazor X system is its robotic arm, which is securely attached to the side of the operating table. The arm, mounted on the patient’s body, is guided to a precise location by 3D optical tracking cameras. In addition, the robotic arm is equipped with 3 integrated linear optical cameras that allow the robot to perform a volumetric assessment of the work environment. This feature enables the robot to self-detect its location and avoid intraoperative collisions. Moreover, the Mazor X system allows independent registration of each vertebra [13].
The latest technological breakthrough in intraoperative robotics is the introduction of real-time image guidance and navigation techniques. This led to the development of the Mazor X Stealth Edition technology in 2019, which combines the existing features of Mazor X with Medtronic’s Stealth intraoperative navigation software. The Mazor X Stealth Edition is equipped with 3D cameras, fiducial guidance markers, and a robotic arm that accurately tracks the location of tools and implants in relation to the spine (Fig. 1).
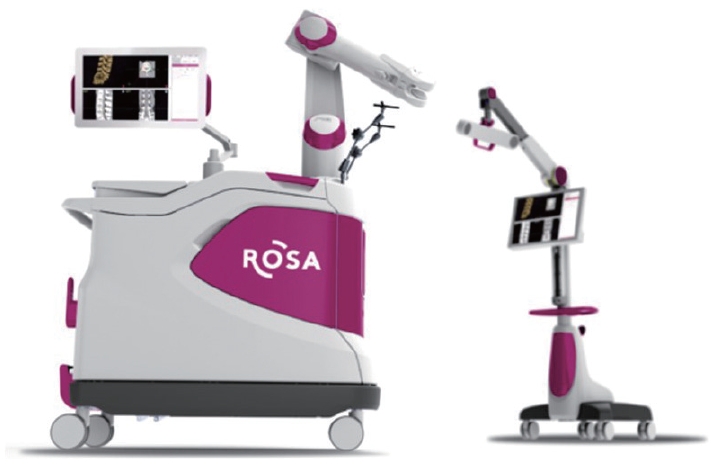
The ROSA surgical robot (Medtech, Zimmer-Biomet, Warsaw, IN, USA) represents another significant advancement in the field of robotic-assisted spinal surgery, having received FDA approval in 2016 [14]. As a freestanding robotic spinal surgical navigation system, the ROSA is composed of a robotic arm, navigation camera, and floor-fixable mobile base. The process of using the ROSA begins with the use of preoperative computed tomography (CT) scan images. A percutaneous reference pin is placed on the iliac wing to confirm location. Subsequently, a fiducial box is attached to the robotic arm. A crucial step in this process involves 3D reconstruction after inspection of the CT images. This is followed by the merging of preoperative and intraoperative CT scans to create a comprehensive operative plan. In 2019, the system was upgraded to the ROSA One spine system, which subsequently received FDA approval. This upgrade brought about full integration with the navigation interface and Zimmer Biomet instrumentation package, further enhancing the capabilities of this innovative robotic guidance system for spinal surgery (Fig. 2).
The ExcelsiusGPS robotic guidance and navigation system (Globus Medical Inc., Audubon, PA, USA) represents a significant development in the field of spinal surgery, having received FDA approval in 2017 [12,15]. This system uses a floor-mounted robotic arm along with fiducial and surveillance markers placed on the posterior superior iliac spine. A significant advantage of the ExcelsiusGPS system is its ability to incorporate pre- or intraoperative CT images, which aid in the guidance of a rigid robotic arm. This, in turn, allows for real-time visualization of instrument positioning and screw placement relative to the patient’s anatomy. The system is equipped with advanced sensors that can detect drill skiving or sliding in the reference frame. Importantly, these sensors can automatically compensate for patient movements, thereby ensuring that the procedure is performed accurately and effectively (Fig. 3).
The CUVIS-spine (Curexo Inc., Seoul, Korea) is a state-of-the-art surgical robotic system specifically designed to enhance the precision and accuracy of pedicle screw placement during spinal surgery. This innovative system comprises a robotic arm, an optical tracking system (Polaris Vega; NDI, Waterloo, ON, Canada), and a sophisticated real-time surgical planning system [16]. A unique feature of the CUVIS-spine is its ability to utilize intraoperative images to meticulously plan targets for pedicle screw placement. This ensures that the surgical procedure is tailored to the patient’s anatomy, ultimately enhancing the accuracy and precision of the surgery. Moreover, the system is equipped with advanced sensors that provide real-time feedback on the lateral repulsive force when the robot contacts the bone surface. This feedback is invaluable as it enables the surgeon to make informed decisions and adjustments during surgery, thus minimizing risks and improving surgical outcomes. Additionally, the CUVIS-spine is designed to continuously monitor the movement of the patient’s marker. This ensures that any shifts or movements of the patient during surgery are accounted for, and the robotic arm is guided according to the correct target position, further adding to the precision of the system (Fig. 4).
The CirQ robotic arm (BrainLAB AG, Mucich, Germany) received FDA approval in 2019. This robotic arm uses an Airo (BrainLAB AG, Munich, Germany) intraoperative CT cage. CirQ is preoperative imaging, screw planning, intraoperative imaging with automatic registration, fusion of the preoperative and intraoperative imaging with a review of the preplanned screw trajectories, robotic-assisted insertion of K-wires followed by fluoroscopy-assisted insertion of pedicle screws and a control. It is performed in the order of intraoperative computerized tomography [17,18].
TIVAVI (codesigned by Beijing Jishuitan Hospital and Beijing Tinavi Medical Technologies Co., Ltd., Beijing, China) is a third-generation orthopedic surgery robot developed independently in China and has only obtained China Food and Drug Administration approval. TIVAVI uses O-arm combined with a CT 3D real-time navigation system (Stealth Station, Medtronic, Minneapolis, MN, USA) [19]. TIVAVI is used not only for spine surgery but also for minimally invasive internal fixation in nondisplaced navicular fractures and complex pelvic acetabular fractures. TIVAVI consists of a robotic arm system, optical positioning and tracking system, and surgical planning system. The robotic arm is highly flexible and stable and is responsible for surgical planning and path positioning, and the optical tracking system monitors the patient reference frame and the robotic arm position, tracking data in real time. Surgical planning and navigation system collects 3D images through intraoperative O-arm scanning. Through the integration of the robotic arm and navigation system, interaction between surgical planning and execution information occurs. In addition, the optical tracking system detects the patient’s actual position and subtle position changes in real time, and the robotic arm increases the accuracy of screw placement through real-time motion compensation [19,20] (Table 1).
The incorporation of robotic and navigation systems in spinal surgery has brought about transformative advantages such as fundamentally improving surgical precision, reducing invasiveness, and minimizing radiation exposure [21].
Robotic systems provide real-time guidance and 3D imaging, allowing precise placement of pedicle screws and reducing the risk of misplacement that could lead to complications.
The precision afforded by these systems allows for smaller incisions, ultimately resulting in less bleeding and reduced risk of infection. Furthermore, minimal muscle dissection and retraction are required, which contribute to faster recovery time and less postoperative pain.
With the advanced imaging and navigation capabilities of robotic systems, the need for intraoperative fluoroscopy is significantly reduced; thus, radiation exposure to both the patient and the surgical team is minimized.
In addition to these advantages, robotic systems offer unique benefits over human capabilities, including:
• Elimination of hand and wrist fatigue: The robotic arm does not experience fatigue and can maintain a steady operation for extended periods.
• Tremor reduction: Robotic systems can filter hand tremors, ensuring smooth and stable movements.
• Precise repetition: These systems can perform repetitive tasks with exactly the same precision each time, which is especially beneficial for procedures that require a high degree of accuracy.
The use of pedicle screws for thoracolumbar spine fusion has recently increased in spinal surgery. The primary methods for pedicle screw fixation are free-hand and fluoroscopy-based techniques. However, both methods require a substantial learning curve. Instances of anatomical variation, such as a narrow pedicle or rotatory scoliosis, can lead to complications, including dural tears as well as spinal cord and nerve injuries, resulting from pedicle screw misplacement.
The free-hand technique of pedicle screw placement is associated with a misplacement rate ranging from 2%–31% [22-25], while the fluoroscopy-based technique has a misplacement rate between 2% and 22% [26,27]. In most studies, the accuracy of pedicle screw placement is verified postoperatively through CT scans, with a screw position of approximately 1–2 mm within the pedicle, which is generally considered clinically insignificant.
To quantify the accuracy of pedicle screw placement, most studies employ the Gertzbein-Robbins scale, which comprises the following grades [28] (Table 2).
In 2010, a multicenter, retrospective review of 3,271 pedicle screw placements performed using SpineAssist was conducted by Devito et al. [29]. Of the 139 patients (646 pedicle screws) evaluated by CT, 98.3% were in the safe zone, and 89.3% were graded as A, 9% as B, 1.4% as C, and 0.3% as D.
A randomized controlled trial conducted by Hyun et al. [30] compared robot-assisted pedicle screw placement using the Mazor Renaissance with fluoroscopy. The study found no significant difference in accuracy between the 2 methods (robotic vs. fluoroscopy, 100% vs. 98.6%, p = 0.500). However, the distance from the proximal facet was significantly different, at 5.8 mm for robotics and 4.6 mm for fluoroscopy (p < 0.001).
Khan et al. [31] investigated the accuracy of pedicle screw placement using Mazor X in 20 patients and found that 98.7% of the 75 screws were grade A and 1.3% were grade B, and all were within the safe zone. Lonjon et al. [32] compared the free-hand pedicle screw technique to ROSA robotic assistance, with ROSA yielding an accuracy rate of 97.3% compared to 92% for the free-hand technique. This study highlighted the benefits of the ROSA in improving accuracy by adjusting the pedicle entry point and trajectory in real-time based on the continuous monitoring of patient movements.
Chenin et al. [14] investigated the accuracy of screws in minimally invasive transforaminal lumbar interbody fusion (TLIF) (n = 21) and open TLIF (n = 4) using the ROSA spine robot. The results showed 91.8% grade A, 4.5% grade B, 1.8% grade C, and 1.8% grade D with an overall safe zone accuracy of 96.3%. Kim et al. [33] compared the screw accuracy and safety between robot-assisted minimally invasive posterior lumbar interbody fusion (PLIF) and open PLIF. Similar accuracy rates were found between the 2 groups, with 91% grade A, 8% grade B, and 1% grade C for the robot-assisted group and 95% grade A and 5% grade B for the open PLIF group (p = 0.534).
Godzik et al. [34] investigated the accuracy of pedicle screws using the ExcelsiusGPS system and reported a breach rate of 3.4% (4 of 116), resulting in an accuracy rate of 96.4%. Ha et al. [16] conducted a study of 448 pedicle screw placements using the CUVIS-spine system in 113 patients. The accuracy rates were 88.4% for grade A, 9.6% for grade B, 1.6% for grade C, 0.2% for grade D, and 0.2% for grade E, with 97.5% clinically acceptable screws and no neurological complications.
In summary, many reports have demonstrated that robotic systems can improve the accuracy of pedicle screw placement. However, it is important to note that similar accuracy rates can be achieved with fluoroscopy, and the understanding of spinal anatomy and surgeon experience play a crucial role in achieving optimal results.
In spinal surgery, fluoroscopy is commonly used to confirm the location of the surgical site and accuracy of instrumentation; this inevitably leads to radiation exposure. The International Commission on Radiological Protection recommends an upper limit of 5 rem/yr for total body exposure and 50 rem/yr for extremity exposure, advocating minimization of radiation exposure as much as possible [35].
Smith et al. [36] conducted a comparison between C-arm fluoroscopy and computer-assisted image guidance using a cadaver, resulting in exposure of 4.33 ± 2.66 mRem for fluoroscopy and 0.33 ± 0.82 mRem for computer-assisted image guidance, representing a significant reduction in ionizing radiation exposure to the torso of the operating surgeon for computer-assisted image guidance. Additionally, Villard et al. [37] highlighted a 9.96-fold higher accumulated radiation dose for the surgeon in screw placement using navigation (intraoperative 3D fluoroscopy-based) compared to nonnavigated (2-dimensional [2D] fluoroscopy-guided) methods.
The variation in radiation exposure during fluoroscopy is contingent on the expertise and skills of the surgeon and fluoroscopy technician. Although intraoperative CT exposes the patients to a certain degree of radiation, the operator remains unexposed. Most studies favor O-arm intraoperative CT to fluoroscopy for lower radiation exposure, whereas other studies found no significant difference between the two. Tabaraee et al. [38] found that the radiation dose to surgeons was lower with the O-arm than with the C-arm. However, for cadavers, higher radiation exposure was experienced with the O-arm group than with the C-arm group. Mendelsohn et al. [39] conducted a comparison between an intraoperative CT-based group and a 2D fluoroscopy group, demonstrating a decrease in radiation exposure for the surgical team but an increase for the patient in the intraoperative CT-based group compared with the 2D fluoroscopy group.
Furthermore, Abul-Kasim et al. [40] validated that by altering the O-arm scan settings, radiation doses could be reduced 5- to 13-fold without compromising the quality of intraoperative images, owing to changes in image resolution. Lian et al. [41] confirmed that intraoperative helical CT resulted in an average radiation dose of 5.47 mSv (0.547 Rem), thereby promoting a reduction in radiation exposure.
Technological advancements and adjustments in imaging techniques can significantly diminish radiation exposure and safeguard both patients and medical practitioners.
Robotic and navigation systems have been shown to improve the accuracy of procedures; however, the impact of this improved accuracy on clinical outcomes, specifically reduction of neurological nerve injury and rehabilitation rates, requires further exploration. Verma et al. [8] conducted a meta-analysis to compare the clinical outcomes of O-arm-assisted navigation with those of free-hand and/or fluoroscopic-guided screw placement. The results indicated that O-arm-assisted navigation was associated with significant improvements, including a reduction in mispositioned pedicle screws (1.6% vs. 4.2%), shorter hospital stays (4.72 days vs. 5.43 days), and lower spine-related readmission rates (0.8% vs. 4.2%). Moreover, there was a decrease in reoperation rates for hardware failure (2.9% vs. 5.9%), as well as a reduction in all-cause reoperations. Conversely, Watkins et al. [42] reported that while the use of robotic and navigation systems lowered the reoperation rate, the difference was not statistically significant. Furthermore, the study revealed an increase in the costs associated with the use of these advanced systems.
Robotic and navigation systems are innovative tools incorporated into spinal surgery to enhance accuracy and potentially improve clinical outcomes. However, these systems involve a significant learning curve and are associated with substantial costs, which raises questions regarding their overall effectiveness and cost-efficiency in clinical practice.
The learning curve for robot-assisted spinal surgery is notable. Studies have shown that pedicle screw placement accuracy improves more rapidly with robotic assistance than with traditional free-hand or fluoroscopy-guided techniques. However, the exact number of cases and time required to reach proficiency with these systems remains unclear. Hu and Lieberman [43] reported learning curves for screw position accuracy, procedure time, and radiation exposure, with improvements noted up to 150 procedures performed using the SpineAssist robot. Similarly, Hyun et al. [30] observed reductions in radiation exposure and pedicle screw insertion times after 30 procedures. It is crucial to note that the learning curve applies not only to the surgeon but also to the assistant surgical team, including operating room nurses and radiology technicians.
The installation and maintenance expenses associated with robotic and navigation systems are considerable. For instance, the cost of the Mazor Renaissance ranges from $700,000–800,000, the Mazor X is approximately $1.5 million, the ROSA is $700,000, and the Excelsius GPS is $1.5 million. Menger et al. [44] reported that robotic spinal surgery is cost-effective considering factors such as fewer revision surgeries, lower infection rates, reduced hospital stays, and shorter operative times. Conversely, in a study involving 360 matched patients, Passias et al. [45] found that robot-assisted surgery was associated with more complications and greater costs than traditional methods.
In conclusion, although robotic and navigational systems offer promise for improved accuracy and potential benefits for clinical outcomes, further research is required to fully assess their cost-effectiveness. Future studies should consider the training costs, variable initial expenses, maintenance and service fees, and operating costs of these advanced platforms.
The use of robotic and navigation systems in spinal surgery has expanded beyond pedicle screw placement to include a variety of spinal procedures. These include deformity correction, iliac and S2 alar-iliac screw placement, interbody fusion, pediatric surgery, and cervical spinal surgery. Software improvements aid in the precision of screw head alignment, as well as in determining screw length and width.
The accuracy enhanced by the navigation systems during S2 alar-iliac and iliac screw insertion improves pelvic obliquity correction and anchor stability. This reduces the surrounding tissue damage, bleeding, and wound infection [46,47].
Joseph et al. [48] evaluated the position of the cages in lateral lumbar interbody fusion using an image-guided navigation system. Their study, which included 66 levels, demonstrated that the navigation systems improved the safe and accurate placement of cages in the anterior or middle portion of the disc space, thereby reducing complications and radiation exposure. Moreover, navigation systems are becoming increasingly popular for long-segment constructs, high-grade spondylolisthesis, pseudoarthrosis, deformity correction, and the resection of metastatic and primary tumors of the spine [4]. These technological advancements have played crucial roles in enhancing the precision, safety, and efficacy of various spinal surgeries.
In conclusion, our review demonstrates that the use of robotic and navigations systems has a significant role to play in the future of spinal surgery. However, despite the clear advantages in terms of clinical outcomes, learning curves, and reduced radiation exposure, the cost-effectiveness of these technologies required further investigation. Therefore, it is crucial to establish clinical efficacy and cost-effectiveness by developing and implementing new instruments and methods.
NOTES
Fig. 1.
The Mazor X robotic system is combined with Medtronic's Stealth O-arm system. Copyright permission obtained from Medtronic.
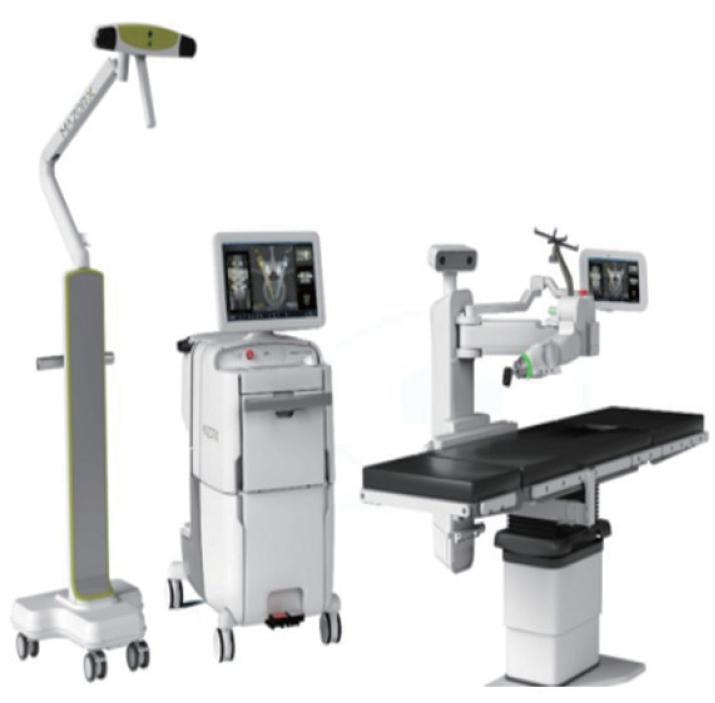
Fig. 4.
(A) The CUVIS-spine system (Curexo Inc., Seoul, Korea) allows surgical instruments and screws to be inserted into the planned path generated by a surgeon using intraoperatively scanned 2-dimensional or 3-dimensional images. (B) The main console and robotic arm make a trajectory for insertion of the screw. Copyright permission obtained from CUREXO Inc.
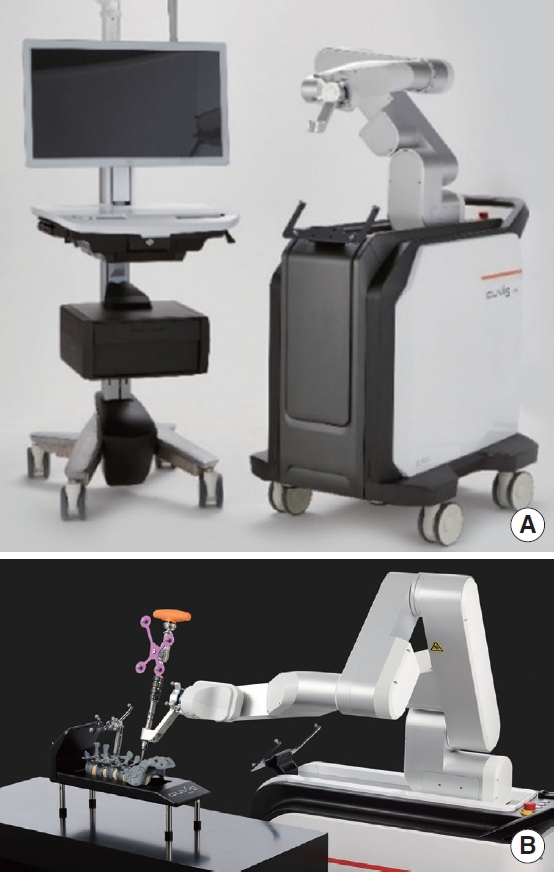
Table 1.
Robotic systems for spinal surgery
REFERENCES
1. Horsley V, Clarke RH. The structure and functions of the cerebellum examined by a new method. Brain 1908;31:45-124.

2. Maciunas RJ, Galloway RL Jr, Fitzpatrick JM, et al. A universal system for interactive image-directed neurosurgery. Stereotact Funct Neurosurg 1992;58:108-13.



3. Zamorano LJ, Nolte L, Kadi AM, et al. Interactive intraoperative localization using an infrared-based system. Stereotact Funct Neurosurg 1994;63:84-8.



4. Perfetti DC, Kisinde S, Rogers-LaVanne MP, et al. Robotic spine surgery: past, present, and future. Spine (Phila Pa 1976) 2022;47:909-21.


5. Vadalà G, De Salvatore S, Ambrosio L, et al. Robotic spine surgery and augmented reality systems: a state of the art. Neurospine 2020;17:88-100.




6. Nathoo N, Cavuşoğlu MC, Vogelbaum MA, et al. In touch with robotics: neurosurgery for the future. Neurosurgery 2005;56:421-33. discussion 421-33.


7. Beutler WJ, Peppelman WC Jr, DiMarco LA. The da Vinci robotic surgical assisted anterior lumbar interbody fusion: technical development and case report. Spine (Phila Pa 1976) 2013;38:356-63.

8. Verma R, Krishan S, Haendlmayer K, et al. Functional outcome of computer-assisted spinal pedicle screw placement: a systematic review and meta-analysis of 23 studies including 5,992 pedicle screws. Eur Spine J 2010;19:370-5.




9. Shin BJ, James AR, Njoku IU, et al. Pedicle screw navigation: a systematic review and meta-analysis of perforation risk for computer-navigated versus freehand insertion. J Neurosurg Spine 2012;17:113-22.


10. Gelalis ID, Paschos NK, Pakos EE, et al. Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur Spine J 2012;21:247-55.




11. Overley SC, Cho SK, Mehta AI, et al. Navigation and robotics in spinal surgery: where are we now? Neurosurgery 2017;80(3S):S86-99.


12. Wang TY, Park C, Dalton T, et al. Robotic navigation in spine surgery: where are we now and where are we going? J Clin Neurosci 2021;94:298-304.


13. D’Souza M, Gendreau J, Feng A, et al. Robotic-assisted spine surgery: history, efficacy, cost, and future trends. Robot Surg 2019;6:9-23.


14. Chenin L, Peltier J, Lefranc M. Minimally invasive transforaminal lumbar interbody fusion with the ROSA(TM) Spine robot and intraoperative flat-panel CT guidance. Acta Neurochir (Wien) 2016;158:1125-8.



15. Elswick CM, Strong MJ, Joseph JR, et al. Robotic-assisted spinal surgery: current generation instrumentation and new applications. Neurosurg Clin N Am 2020;31:103-10.

16. Ha BJ, Lee JM, Yoon SJ, et al. Three-dimensional quantitative assessment of pedicle screw accuracy in clinical utilization of a new robotic system in spine surgery: a multicenter study. Neurospine 2023;20:1028-39.




17. Pojskić M, Bopp M, Nimsky C, et al. Initial intraoperative experience with robotic-assisted pedicle screw placement with Cirq(®) robotic alignment: an evaluation of the first 70 screws. J Clin Med 2021;10:5725.



18. Chesney K, Triano M, Dowlati E, et al. Cirq robotic arm-assisted transpedicular instrumentation with intraoperative navigation: technical note and case series with 714 thoracolumbar screws. J Robot Surg 2022;16:893-8.



19. Li S, Du J, Huang Y, et al. Comparison of surgical efficacy between O-arm combined with CT 3D real-time navigation system and Tinavi robot-assisted treatment of adolescent congenital scoliosis. Am J Transl Res 2023;15:3254-66.


20. Du J, Gao L, Huang D, et al. Radiological and clinical differences between robotic-assisted pedicle screw fixation with and without real-time optical tracking. Eur Spine J 2021;30:142-50.



21. Kochanski RB, Lombardi JM, Laratta JL, et al. Image-guided navigation and robotics in spine surgery. Neurosurgery 2019;84:1179-89.


22. Kim YJ, Lenke LG, Bridwell KH, et al. Free hand pedicle screw placement in the thoracic spine: is it safe? Spine (Phila Pa 1976) 2004;29:333-42. discussion 342.


23. Karapinar L, Erel N, Ozturk H, et al. Pedicle screw placement with a free hand technique in thoracolumbar spine: is it safe? J Spinal Disord Tech 2008;21:63-7.


24. Modi HN, Suh SW, Hong JY, et al. Accuracy of thoracic pedicle screw using ideal pedicle entry point in severe scoliosis. Clin Orthop Relat Res 2010;468:1830-7.



25. Parker SL, McGirt MJ, Farber SH, et al. Accuracy of free-hand pedicle screws in the thoracic and lumbar spine: analysis of 6816 consecutive screws. Neurosurgery 2011;68:170-8. discussion 178.



26. Suk SI, Kim WJ, Lee SM, et al. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine (Phila Pa 1976) 2001;26:2049-57.

27. Beck M, Mittlmeier T, Gierer P, et al. Benefit and accuracy of intraoperative 3D-imaging after pedicle screw placement: a prospective study in stabilizing thoracolumbar fractures. Eur Spine J 2009;18:1469-77.




28. Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976) 1990;15:11-4.


29. Devito DP, Kaplan L, Dietl R, et al. Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine (Phila Pa 1976) 2010;35:2109-15.

30. Hyun SJ, Kim KJ, Jahng TA, et al. Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine (Phila Pa 1976) 2017;42:353-8.

31. Khan A, Meyers JE, Siasios I, et al. Next-generation robotic spine surgery: first report on feasibility, safety, and learning curve. Oper Neurosurg (Hagerstown) 2019;17:61-9.


32. Lonjon N, Chan-Seng E, Costalat V, et al. Robot-assisted spine surgery: feasibility study through a prospective casematched analysis. Eur Spine J 2016;25:947-55.



33. Kim HJ, Lee SH, Chang BS, et al. Monitoring the quality of robot-assisted pedicle screw fixation in the lumbar spine by using a cumulative summation test. Spine (Phila Pa 1976) 2015;40:87-94.


34. Godzik J, Walker CT, Hartman C, et al. A Quantitative assessment of the accuracy and reliability of robotically guided percutaneous pedicle screw placement: technique and application accuracy. Oper Neurosurg (Hagerstown) 2019;17:389-95.


36. Smith HE, Welsch MD, Sasso RC, et al. Comparison of radiation exposure in lumbar pedicle screw placement with fluoroscopy vs computer-assisted image guidance with intraoperative three-dimensional imaging. J Spinal Cord Med 2008;31:532-7.



37. Villard J, Ryang YM, Demetriades AK, et al. Radiation exposure to the surgeon and the patient during posterior lumbar spinal instrumentation: a prospective randomized comparison of navigated versus non-navigated freehand techniques. Spine (Phila Pa 1976) 2014;39:1004-9.

38. Tabaraee E, Gibson AG, Karahalios DG, et al. Intraoperative cone beam-computed tomography with navigation (O-ARM) versus conventional fluoroscopy (C-ARM): a cadaveric study comparing accuracy, efficiency, and safety for spinal instrumentation. Spine (Phila Pa 1976) 2013;38:1953-8.

39. Mendelsohn D, Strelzow J, Dea N, et al. Patient and surgeon radiation exposure during spinal instrumentation using intraoperative computed tomography-based navigation. Spine J 2016;16:343-54.


40. Abul-Kasim K, Söderberg M, Selariu E, et al. Optimization of radiation exposure and image quality of the cone-beam O-arm intraoperative imaging system in spinal surgery. J Spinal Disord Tech 2012;25:52-8.


41. Lian X, Navarro-Ramirez R, Berlin C, et al. Total 3D Airo® Navigation for Minimally Invasive Transforaminal Lumbar Interbody Fusion. Biomed Res Int 2016;2016:5027340.




42. Watkins RG, Gupta A, Watkins RG. Cost-effectiveness of image-guided spine surgery. Open Orthop J 2010;4:228-33.




43. Hu X, Lieberman IH. What is the learning curve for robotic-assisted pedicle screw placement in spine surgery? Clin Orthop Relat Res 2014;472:1839-44.



44. Menger RP, Savardekar AR, Farokhi F, et al. A cost-effectiveness analysis of the integration of robotic spine technology in spine surgery. Neurospine 2018;15:216-24.




45. Passias PG, Brown AE, Alas H, et al. A cost benefit analysis of increasing surgical technology in lumbar spine fusion. Spine J 2021;21:193-201.


46. Kim KD, Duong H, Muzumdar A, et al. A novel technique for sacropelvic fixation using image-guided sacroiliac screws: a case series and biomechanical study. J Biomed Res 2019;33:208-16.



- TOOLS
-
METRICS

-
- 2 Crossref
- Scopus
- 2,025 View
- 115 Download
-
Journal Impact Factor 3.8
SURGERY: Q1
CLINICAL NEUROLOGY: Q1